| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
원료 ≫ 기능성 ≫ 비타민, 미네랄 비타민의 산업적 생산 : 화학합성 생합성 - 비타민C : 천연합성 & 화학합성 - 비타민 B12 https://en.wikipedia.org/wiki/Vitamin_B12_total_synthesis Riboflavin (B2) can be produced by Ashbya gossypii, Eremothecium ashybii Cobalamine (B12) cause produced by bacteria propioni bacterium, Pseudomonas denitrificans, Bacillus megatherium, Streptomyces oleaceus 비타민 C : 포도당 hydrogenation of D-glucose to D-sorbitol, an organic reaction with nickel as a catalyst under high temperature and high pressure. Microbial oxidation or fermentation of sorbital to L-sorbose with acetobacter [1] with pH 4-6 and 30 °C. protection of the 4 hydroxyl groups in sorbose by formation of the acetal with acetone and an acid to Diacetone-L-sorbose (2,3:4,6−Diisopropyliden−α−L−sorbose) Organic oxidation with potassium permanganate followed by heating with water gives the 2-Keto-L-gulonic acid The final step is a ring-closing step or gamma lactonization with removal of water .[2] Intermediate 5 can also be prepared directly from 3 with oxygen and platinum 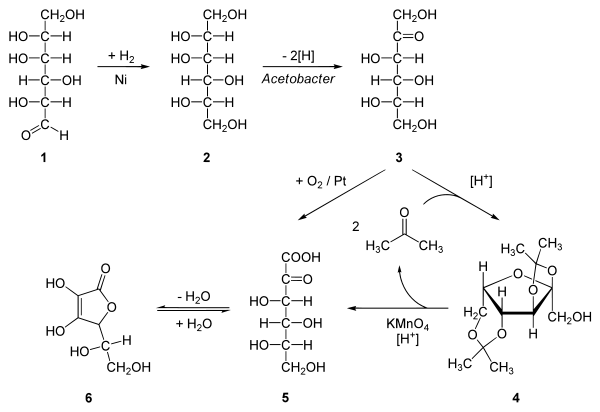 비타민 E : myrcene The chlorination of myrcene (I) with Cl2 in refluxing pentane gives the choromyrcene (II), and the hydrochlorination of (I) catalyzed by CuCl yields a mixture of geranyl/neryl chloride (III). The reductive coupling of (II) and (III) by means of Mg and CuCl affords beta-springene (IV), which is condensed with 2,3,6-trimethylhydroquinone (V) by means of cyclooctadienyl rhodium chloride dimer [RhCl(COD)]2 and K2CO3 in refluxing toluene to provide the adduct (VI). The cyclization of (VI) by means of MeAlCl2 of Ts-OH in refluxing hexane furnishes the tocotrienol (VII), which is finally hydrogenated with H2 over Pd/C in ethanol to give the target (rac)-vitamin E.  Riboflavin (vitamin B2) Various biotechnological processes have been developed for industrial scale riboflavin biosynthesis using different microorganisms, including filamentous fungi such as Ashbya gossypii, Candida famata and Candida flaveri, as well as the bacteria Corynebacterium ammoniagenes and Bacillus subtilis.[39] The latter organism has been genetically modified to both increase the bacteria's production of riboflavin and to introduce an antibiotic (ampicillin) resistance marker, and is now successfully employed at a commercial scale to produce riboflavin for feed and food fortification purposes. The chemical company BASF has installed a plant in South Korea, which is specialized on riboflavin production using Ashbya gossypii. The concentrations of riboflavin in their modified strain are so high, that the mycelium has a reddish/brownish color and accumulates riboflavin crystals in the vacuoles, which will eventually burst the mycelium. Riboflavin is sometimes overproduced, possibly as a protective mechanism, by certain bacteria in the presence of high concentrations of hydrocarbons or aromatic compounds. One such organism is Micrococcus luteus (American Type Culture Collection strain number ATCC 49442), which develops a yellow color due to production of riboflavin while growing on pyridine, but not when grown on other substrates, such as succinic acid.[40] Niacin (L) of potassium permanganate oxidation of nicotinic acid which is the earliest methods of production to 3 - methyl-pyridine as raw material by potassium permanganate oxidation of nicotinic acid. Products slightly red, and inconsistent quality; material 3 - methylpyridine low conversion rate (85%); greater consumption of potassium permanganate (lt 2.5t niacin consumption of potassium permanganate.) The high cost method, equipment corrosion larger, not suitable for industrial production. (2) nitric acid oxidation of nitric acid as oxidant, the mixture of nitric acid solution and pass into the titanium tube MEP reactor, controlling the reaction temperature is 330 ℃, pressure of 29MPa, reaction of 8h, after purified by the separation of nicotinic acid. This method requires a higher temperature, the reaction selectivity, serious equipment corrosion, low yield, waste pollution is serious, color and poor quality products. (3) ozone oxidation method quinoline dissolved in nitric acid solution, the temperature to 40-50 ℃, which leads to ozone formation of ozone quinoline compounds obtained reflux temperature of 2,3 - dipyridyl acid, nicotinic acid and then decarboxylation get. The method is compared with the previous two methods has certain advantages, no equipment corrosion, waste less, low labor intensity, but more expensive ozone, an increase of production costs, the need to build another ozone plant, equipment investment is large, is not suitable large-scale industrial production. (4) gas phase oxidation of gas phase oxidation of air or oxygen-enriched air is the oxidant oxidation of 3 - methyl-pyridine system nicotinic acid. This method was first air Walter alkyl pyridine with the catalyst in the reaction solution, later changed to 3 - methyl-pyridine gasification together with air into the tube reactor, reaction temperature 350-400 ℃, reaction time 3h. Catalyst for the long term applied to oxygen as the oxidant, the oxidation process step can be completed, is a cost-efficient production process. This reaction is the key to select the appropriate catalyst, difficulties still exist, and the reaction temperature is high, yet there are reports of industrialization. (5) electrolytic oxidation is the production of electrolytic oxidation method is widely used as a method of application conditions with mild oxidant is relatively cheap, toxic pollution, less waste, lower production cost advantages, but the electrolysis efficiency of such reactions is low, mainly because the cell membrane permselectivity used in isolation is poor, so that the method for industrial production are more restricted. (6) of the Act hydroxide method of 3 - methylpyridine or MEP as raw materials, and ammonia, by a certain percentage of oxygen in the catalyst under the oxidation reaction to produce smoke nitrile, hydrolysis in alkaline solution and subsequently by the purified nicotinic acid. The reaction carried out under normal pressure or low pressure with high yield, high purity products, safe and reliable product, suitable for continuous large-scale industrial production, industrial preparation of nicotinic acid is widely used abroad, the process route. 비타민 D3 Here are the simplified steps for Vitamin D3 production: •Depending on the breed, healthy sheep will produce from 2 to 30 pounds of wool each year. •Wool is sheared from mature, live sheep. •Crude lanolin is extracted from the wool using a scouring process, during which the fleece is washed in hot water with a detergent. •Crude lanolin undergoes a saponification process; this separates the fatty component which is removed via centrifugation, from the ‘unsaponifiable’ component, known as ‘lanolin alcohols’. These undergo further steps of saponification and separation to increase purity. •Crude cholesterol is extracted from lanolin alcohol using solvent washes and/or column chromatography. •The crude cholesterol undergoes a series of further solvent extractions, washes and drying until it is extremely pure and crystalline. •Purified cholesterol is then taken through a four-step chemical process to make 7-Dehydrocholesterol, this is otherwise known as ‘pre-Vitamin D3’ •Next, the pre-Vitamin D3 is irradiated to produce Vitamin D3 (cholecalciferol); this is the same reaction that is used by human skin to manufacture the vitamin from sunlight. Some unwanted isomers are formed during irradiation. These are removed by various techniques, leaving a resin which melts at about room temperature and usually has a potency of 25,000,000 to 30,000,000 International Units per gram. Finally, the pure crystals of Vitamin D3 are used to make the stabilized product forms that can be used to manufacture dietary supplements and other end use applications such as foods and beverages. 비타민 A : citral -orange |
||||
|
|
|||