|
단백질 ≫ 단백질종류 ≫ 콜라겐
콜라겐의 합성 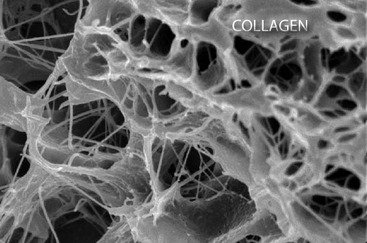
콜라겐: 세포골격, ECM
- 증점(단순)다당류 , 복합다당류
- 세포는 Fiber 덩어리다 ,
- 엘라스틴 elastin, Proteoglycan
콜라겐 조성 및 합성과정
- 콜라겐 역할 : 세포골격
- 콜라겐 역할 : 세포 생명유지 & 세포자살
- 콜라겐 조성 및 합성과정
- 콜라겐 : 결합조직 질환
- 콜라겐 -> 젤라틴
- 비타민 C 역할
* 콜라겐 구성 아미노산 : glycine(27%), proline(15%), glutamic acid(11%), alanin(10%), aginine(8%).
* Elastin 구성 아미노산 : glycine(27%), alanine(21%), valine(18%), proline(14%), leucine(9%)
비필수 아미노산으로 생성
1. 글리신(glycine) : 1/3
2. 프롤린 + 하이드록시프롤린 = 30%,
glutamic acid → 프롤린(proline) → Hydroxyproline (Hyp)
3. 라이신 + 하이드록시라이신
라이신(lysine) → Hydroxylysine (Hyl)
프롤린(proline)과 라이신(lysine)에 -OH를 붙이는 과정에서 비타민 C가 필요하다.

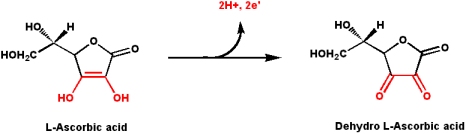
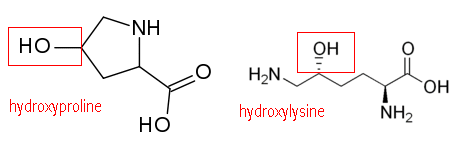

3개의 폴리펲타이드가 나선형 구조로 꼬이기 전의 상태를 프로콜라겐(procollagen)이라 부르고, 이것이 콜라겐으로 전환된다. 콜라겐이 만들어지는 5단계의 각 단계마다 비타민C가 요구된다. 라이신(lysine)과 프롤린(proline)은 ‘수산화반응’에 의해 수산화라이신과 수산화프롤린(hydroxylysine, hydroxyproline)으로 바뀐다. 3개의 폴리펲타이드 체인(triple helix)는 수산화된 라이신과 프롤린을 통해 서로 교차결합(cross-link)되면서 튼튼한 콜라겐이 만들어진다.

5단계를 통해 만들어지는 콜라겐
콜라겐을 구성하는 폴리펲타이드 체인이 다른 단백질과 확연히 다른 구별되는 것은, 이 체인이 글리신과 프롤린의 두 아미노산만으로 서로 교차하면서 중간에 간간이 라이신 등 다른 아미노산이 구두점처럼 끼여 있다는 점이다. 콜라겐은 3차원 구조의 거대분자인데, 3개의 폴리펲타이드 체인이 흡사히 로우프의 꼬인 끈(strand)처럼 나선형(helix)구조로 서로 꼬여 콜라겐이라는 섬유(fibril)단백질이 된다.
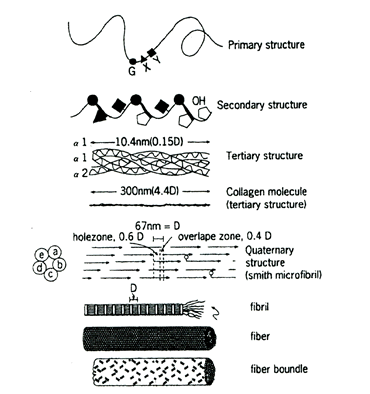 

콜라겐은 그대로 흡수될 수가 없다. 콜라겐은 아미노산으로 잘게 쪼개진 후에만 흡수될 수 있다. 이 말은 콜라겐을 충분하게 그리고 튼튼하게 만들기 위해서는, 인체가 콜라겐을 잘 만들 수 있도록 원료와 촉진물질(촉매, 효소)을 원활하게 공급해야 한다는 것을 의미한다.(콜라겐을 직접 먹는 것은 별다른 의미가 없다)
OH기를 치환하는 이유 : Hydrogen bond
- Hydrogen bond : 생명현상에서 가장 중요한 결합이다
가장 평범한 아미노산을 이용 : 라이신, 프롤린
-OH를 붙이면 강력한 Hydrogen bond로 단단한 구조체를 만들수 있다



Self-assembly into Supramolecular Structure 체온에서 자발적인 조립
The collagen triple helix is essentially a one-dimensional molecule, with its near-constant axial separ between amino acid residues throughout the triple-helical domain. This has permitted a direct correla structural data obtained by electron microscopy with chemical sequence data. Lateral association of 1 collagenous triple-helical domains, to form fibrils, is a common feature of the collagen family. Fibril self-assemble spontaneously from solutions of extracted type I collagen when the pH, temperature, a strength are adjusted to physiological values. This lateral association appears to be promoted by elev temperature, suggesting that hydrophobic interactions are the principal driving force (Fig. 6). Fibri formation is controlled to a large extent by the amino acid sequence of the collagen and, in particular distribution of polar and hydrophobic residues that are exposed on the surface of the triple-helical do Hydroxylysine and glycosylated hydroxylysine residues might be a most potent candidate involved i limiting the lateral growth of the collagenous domains. The residues on the surface of collagen triple may well be bulky enough to cause steric hindrance in the lateral association of the triple-helical dom (Fig. 15, see later). The situation would be particularly pronounced in the triple-helical domains of ty and type IV collagens, which respectively contain more than 20 and 50 glycosylated hydroxylysine r in a single polypeptide.

Figure 6. Lateral association of triple-helical domains. Triple-helical domain is highly hydrated at 4°C. At physiological temperature such as 37°C, water molecules on the hydrophobic domains will be dehydrated. Collagen molecules accomp lateral association of the triple-helical domains through the exposed hydrophobic regions.
Lateral association of triple-helical domains. Triple-helical domain is highly hydrated at 4°C. At physiological temperature such as 37°C, water molecules on the hydrophobic domains will be dehydrated. Collagen molecules accomp lateral association of the triple-helical domains through the exposed hydrophobic regions.

콜라겐의 생합성







|