Chemistry ≫ pH
산도(acidity)와 pH
- 미생물 : 미생물 생육조건
- 보존료, 음료살균조건
- 해리도 : 강산, 약산
- 산도(acidity)와 pH
- 내산성 : pH는 용해도를 바꾼다
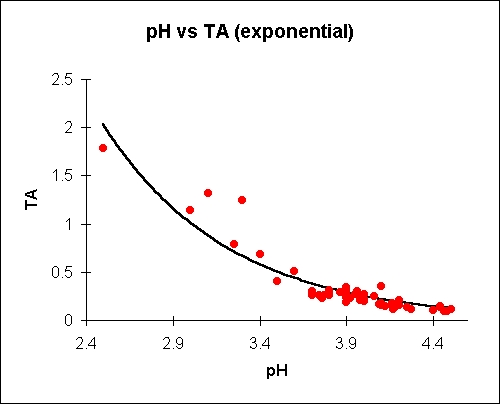
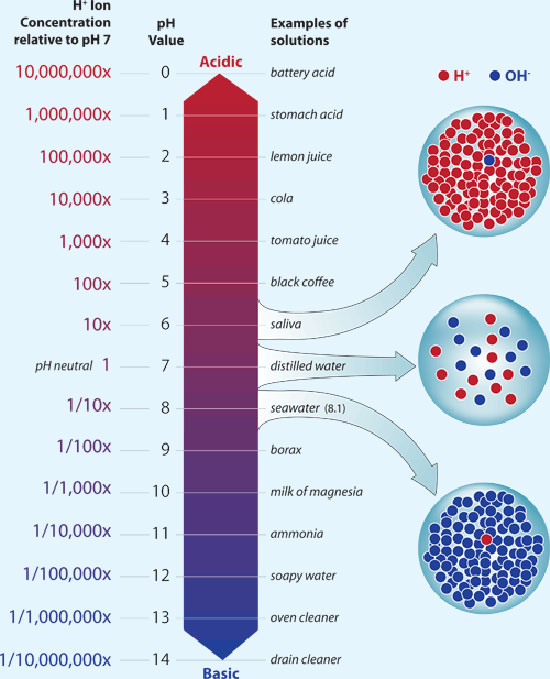
The pH is a logarithmic measure of the concentration of free hydrogen ions in a chemical or biological system. It's a very important concept in cell biochemistry. Titratable acid, on the other hand, is a simple measure of the (related) amount of acid 'anions' in a juice. There is no direct relationship between titratable acidity and pH in apple juice, although generally the pH goes up as the acid goes down and vice-versa. The exact relationship differs from sample to sample and depends on esoteric concepts like 'buffering capacity' which will vary for a whole host of reasons.