갈변 : 색변화, 산화
Chemistry ≫ 온도, pH
갈변 : 색변화, 산화
갈변 : 색변화, 산화
- 갈변 : 멜라로이딘 melanoidin
- 효소적 갈변 : 갈변 방지 GMO
- 비효소적 갈별 : 카라멜반응 Caramelization, 마이야르 반응 Maillard
- 카라멜 반응
- 산소의 독성
- 수분활성도

Maillard reaction is a non-enzymatic browning reaction, caused by the condensation of an amino group and a reducing compound, resulting complex changes in biological and food system. This reaction was described for the first time by Louis Maillard in 1912. Maillard reaction occurs when virtually all foods are heated, and also occurs during storage. Most of the effect of Maillard reaction, including the caramel aromas and golden brown colors, are desirable. Nevertheless, some of the effect of Maillard reaction, including foods darkness and off-flavor development, are undesirable.
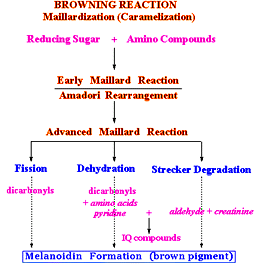
Controlling Factors of the Maillard Reaction Products
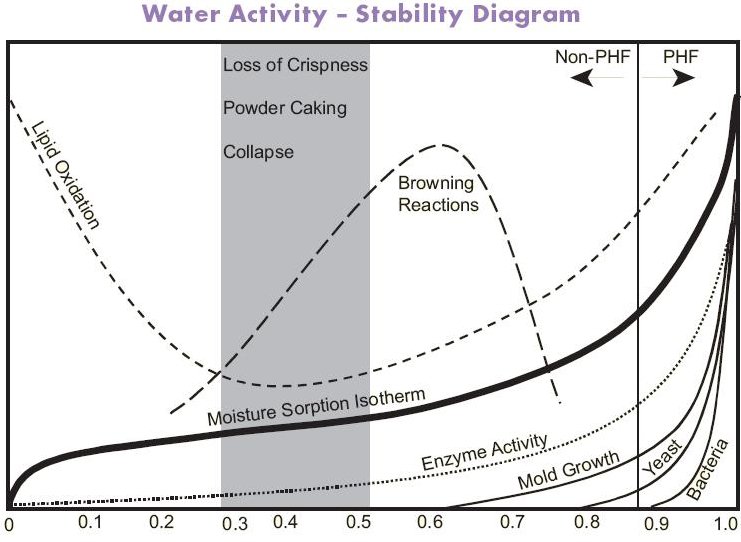
* 수분활성도 Water activity (aw)
Water is produced during Maillard reaction, thus the reaction occurs less readily in foods with a high aw values while, at low aw, the mobility of reactants is limited, despite their presence at increased concentrations.
As the figure shown, in practice the Maillard reaction occurs most rapidly at intermediate aw values (0.5-0.8), and aw is of most significance to the reaction in dried and intermediate- moisture foods (IMFs), which have aw values in this range. However, aw values for maximum browning are affected by other components of the system: humectants, such as glycerol, can lower the aw value for maximum browning.
* pH
Since the reaction itself has a strong influence on pH it is hard to evaluate the pH influence. However, reactions take place by all pathways, with the pH of the system influencing the ratio of products formed and the rate of color formation can be reduced by decreasing the pH. The most desirable meaty and pot-roasted aroma was obtained at pH 4.7, but pH had a less dramatic effect on aroma than did temperature, time or water content.
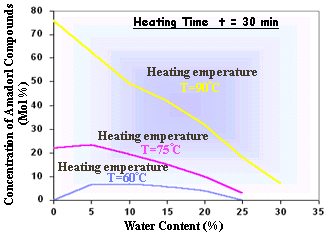
* Temperature
The temperature dependence of chemical reaction is often expressed as the activation energy (Ea). Activation temperature data for the Maillard reaction have been reported within a wide range 10-160KJ/mole, depending on, what effect of the reaction has been measured. The activation energy is also highly dependent on pH. The temperature dependence of the Maillard reaction is also influenced by the participating reactants. So it is difficult to isolate the effect of temperature as a single variable.
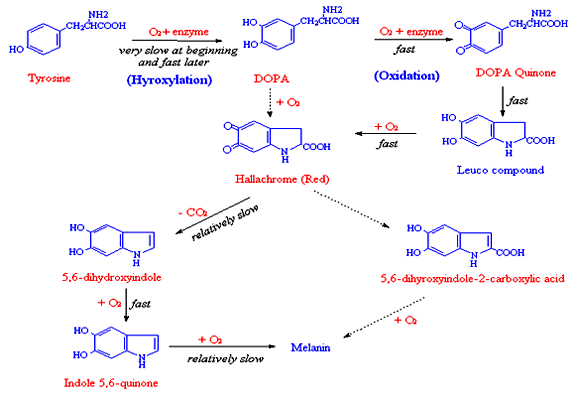
Enzymatic (Oxidative) Browning
Introduction
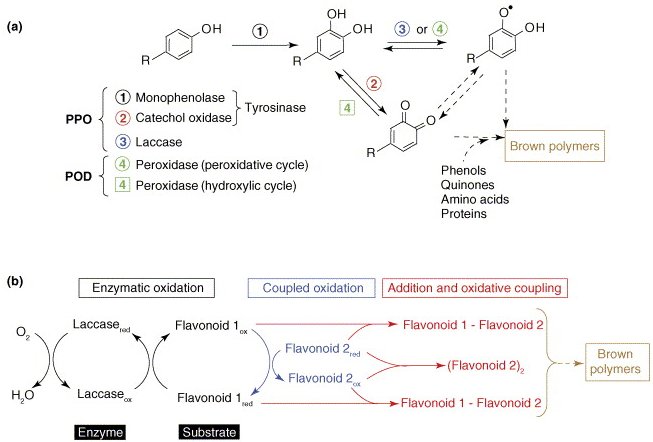
There are four types of browning reactions in foods: Maillard, caramelization, ascorbic acid oxidation and phenolase browning. The former three are non enzymatic in nature, and the phenolase or enzymatic-catalytic oxidation browning is commercial significance, particularly in fruit and vegetables where phenolase are very common. Enzymatic browning is rare at intact tissue, since phenolic substrates and phenolase are separated. Enzymatic browning is very common at the cut surface of light-colored fruits and vegetables. The cut surface may rapidly change to brown color due to the oxidation of phenols to orthoquines, which in turn polymerize to form brown pigment or melanins quickly. Enzymes that catalyze the phenols oxidation may be classified as phenolase, which are oligomers in food and contain one copper prosthetic group per subunit.
Reaction
Two types of reaction are involved in the phenolase catalytic reaction, e.g.. hyroxylation and oxidation (following figure). Hydroxylation of monophenols is slow and rate-determining step in reaction. Besides tyrosine, major substrates of phenolases in plant include caffeic acid, chlorogenic acid and protocatechuic acid which are abundant in plants. But tyrosine and chlorogenic acid are the two most prevalent substrates for phenolase due to their relatively high reaction rates. Phenolase is active in pH5-7, and may be irreversibly inactivated at pH lower than pH 3.

------------

아황산 영향
