GPCR C형 : (단맛, 감칠맛)
감각기관 ≫ 수용체 ≫ GPCR
GPCR C형 : (단맛, 감칠맛)
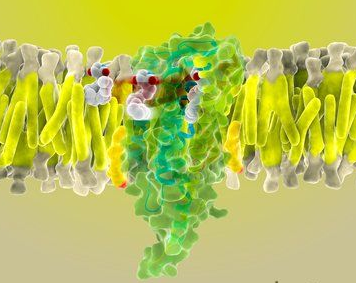
G protein & 감각 기관
- 미각 수용체 : 미각 메커니즘
- 단맛의 기작
- 감칠맛의 기작
먼저 통상적인 7TM에 orthosteric ligand 인식이 가능한 거대한 VFT 영역이 추가된 형태이며 더구나 단맛과 감칠맛은 CLASS C가 2개가 합쳐서 작동한다. class A에 비하여 4배 이상 큰 단백질이 1가지 감각에 사용되는 셈이다. 이 구조는 여러 조절 위치를 가지고 있어서 다양한 활성화 조절 매커니즘이 가능하다. 억제의 조절물질은 결국 7TM 부위가 활성화된 형태도 변화되지 않도록 불활성화된 상태로 그대로 구조를 안정화 시키는 것이고, 활성화 물질은 리간드가 강하게 결합 가능한 형태로 상태를 만들어 주는 물질이다. 이런 조절 가능 부위가 여러 가지 발견되었다. 이것은 약의 개발 등에도 그대로 적용되는 원리이다
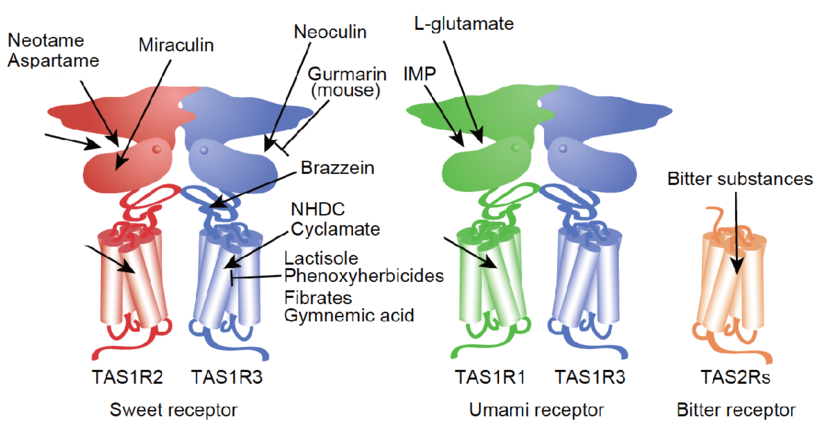
The class C GPCRs contain three taste receptor subunits (T1R1, T1R2, and T1R3) that form two heterodimers, the sweet receptor (T1R2/T1R3) and the umami receptor (T1R1/T1R3)29, 30. Only cis activation occurs within the sweet and umami taste receptors, which means T1R2 in the sweet receptor or T1R1 in the umami receptor are involved in both orthosteric ligand recognition and in G protein activation, whereas the common subunit T1R3 loses the corresponding function31. In addition to natural sugars, the sweet taste receptor is also sensitive to artificial sweeteners, sweet proteins and some D-amino acids. In most mammals, the umami receptor can be activated by L-amino acids, whereas the human orthologue is only sensitive to monosodium glutamate and L-aspartate. Flavor enhancers, such as purine nucleotides, have the ability to potentiate umami receptor function. These artificial sweeteners and flavor enhancers represent a large food sector market6.
Class C GPCRs contain multiple ligand interaction sites as a result of their particularly complicated structure and activation mechanism. These ligand binding sites are divided into two groups: orthosteric and allosteric binding sites. The endogenous ligand binding sites, or orthosteric sites, reside in the VFT domain. Both competitive agonists and antagonists interact with this site and induce significant conformational changes in the VFT: binding of a full agonist stabilizes a closed conformation35, whereas binding of competitive antagonists stabilizes an open conformation33, 34, 43. Binding of partial agonists results in a partial or a complete, yet unstable, closure of the VFT domain35, 58. In contrast, the allosteric sites are topographically distinct from the orthosteric sites in any given receptor. The binding of allosteric modulators changes the receptor conformation and, thereby, the affinities and/or efficacies of orthosteric ligands. In general, the positive allosteric modulators (PAMs) facilitate the action of the orthosteric agonists, whereas the negative allosteric modulators (NAMs) block the activation of orthosteric agonists by stabilizing the 7TM in an inactive conformation. The large extracellular domain and constitutive dimerization of class C GPCRs provide more potential allosteric sites compared with other GPCRs. To date, there are three groups of allosteric sites in class C GPCRs that have been reported (Figure 3).
Taste receptors — multiplicity of various ligand-binding sites
A unique characteristic of taste receptors is their diversity of ligand-binding sites. Aside from the orthosteric sites, there are at least eight allosteric sites that have been identified in taste receptors: the EDAM sites for IMP in T1R1-VFT31 and for SE-2/SE-3 in T1R2-VFT67; the allosteric agonist sites for sweet proteins in T1R3-CRD45, cyclamate in sweet receptor T1R3 7TM70, S807 in T1R1 7TM31 and S819 in T1R2-7TM31; the PAM site for cyclamate in the umami receptor T1R3-7TM70; and the NAM site for lactisole in both the sweet and umami receptor T1R3-7TM71. These sites might represent potential targets for health-related products or drugs to treat diseases, such as hypertension or diabetes.