법칙 ≫ 복합작용 ≫ 상승작용
상승효과 Synergy
- 점도 상승효과 : 증점제간의 상호결합
- 감미 상승효과 : 대비효과, sugar, salt
- 풍미 상승효과 : sugar, acid, flavor
증점제의 상승작용
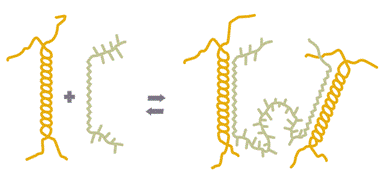
- LBG + Xanthan: 증점제 + 증점제 --> Soft gel --> 강력한 점도 상승작용
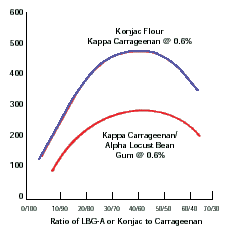
- LBG + 카라기난
In many food systems, there are valid reasons for using combinations
of hydrocolloids to enhance stability and/or texture. For example,
ice cream has a two-part stabilizer system. The primary stabilizer
controls the growth rate of ice crystals during the freezing process
and slows ice crystal growth during the freeze/thaw cycles experienced
during transport and storage. A typical primary stabilizer contains one
or more of the following hydrocolloids: LBG, guar gum, cellulose gum,
and gelatin. The choice between these gums is based on several factors,
including cost-in-use, whether the product is “natural,” whether the
product is kosher, and whether freedom from off-flavors is important.
The primary stabilizer controls the rate of ice crystal growth, but
it also gives the ice cream mix a tendency toward whey separation
(“whey-off”). The secondary stabilizer, carrageenan, provides a very
slight gel to the mix, which is sufficient to stop whey separation.
Table 3-6 is a compatibility chart of the hydrocolloids. This chart
should be used only as a general guide because there are exceptions to
every rule in food hydrocolloid chemistry.
In most cases, there is an additive effect when a gelling gum is used
with a thickening gum. Each gum contributes something to the food
system, and the end result reflects the two effects. For example, low
ester pectin and LBG are used in yogurt fruit preparations. The pectin
gives some gel structure (yield point) while the LBG gives some body
(viscosity) and controls syneresis.
In some cases, the combination of two different thickening gums
gives more viscosity than predicted. For example, if a 1% cellulose
gum solution with a viscosity level of 4,000 cP is mixed with an
equal amount of a 1% solution of guar also having a viscosity level
of 4,000 cP, the net result is not 4,000 cP for the mixture. It is, in fact,
closer to 6,000 cP. This provides an economic incentive to use synergistic
gum systems, because the overall level of gum use is lower. Similarly,
xanthan gum gives a synergistic viscosity increase to cellulose
gel and cellulose gum. However, one must be sure to specify cellulasefree
xanthan gum, because it normally does contain a small amount
of cellulase enzyme, which quickly degrades the cellulose gum with a
resultant loss in viscosity.
In a few cases, a synergistic gelation occurs when two hydrocolloids
are combined. The best-known example of this is a mixture of
LBG and xanthan gum. If LBG and xanthan are placed together in
water at sufficient concentration, heated to 70°C (158°F), and allowed
to cool, a rubbery but rigid gel results. As little as one part LBG in 500
parts xanthan (or vice versa) creates a gel if their total concentration
in water is 0.5–1%. Similarly, xanthan gum and Tara gum gel when
combined.
There are cases of a gum changing the gel strength of another gum.
For example, agar has less gel strength in the presence of sodium alginate
or (to a lesser extent) gum karaya. However, agar has more gel
strength in the presence of LBG or (to a lesser extent) cellulose gum.
LBG greatly modifies the texture of a κ-carrageenan gel, making it
more elastic with a higher break strength, less brittle, and less prone
to syneresis. Also, cellulose gel has more viscosity but less thixotropy
in the presence of too much CMC, MC, MHPC, or HPC.
Some other unusual but potentially useful interactions include the
following:
1. Gels made with sodium alginate and high ester pectin are thermally
reversible, whereas gels of alginate alone or high ester pectin
alone are usually not thermally reversible. The gel temperature
of the algin–pectin gel mixture increases as the system’s pH is
lowered.
2. Xanthan gum reduces the reaction of low ester pectin to calcium.
Thus, more calcium is required to gel low ester pectin in the
presence of xanthan gum.
3. Gellan gum has been used in combination with low ester pectin
to “extend” the low ester pectin (i.e., to reduce the use level of
the pectin without sacrificing gel strength).
4. Pectin and gelatin combinations are additive at pH values above
4.5 or below 3.3. At pH 3.4–4.4, precipitation occurs, unless the
system has a high soluble solids content (75% or higher).
5. Low-concentration mixtures of gum arabic and gelatin precipitate
and form a coacervate, which is used by the flavor industry for
encapsulation. Higher concentrations of the two are used by the
confectionery industry to make a pastille with a soft, rubbery texture.
In this case, there is no precipitation because of the crowded
hydration conditions and the resulting lower water activity.
6. Mixtures of gelatin and carrageenan increase gel strength synergistically
in food systems at pH 6.0 or higher. Gelatin and gellan
gum show a similar increase in synergistic gel strength at pH values
above 5.0. At acid pH values (below 5), gelatin precipitates
either of these two anionic hydrocolloids.
7. As a general rule, gelatin is compatible and potentially synergistic
with anionic hydrocolloids when it is negatively charged (pH
values above the isoelectric pH) and coprecipitates with anionic
hydrocolloids when it is positively charged (pH values below the
isoelectric pH). In other words, gelatin can be used only in combination
with anionic hydrocolloids in neutral or near-neutral
pH systems, such as milk or meat products. The exception to this
rule is systems with low water activity and low pH, such as certain
confectionery formulations where gum arabic–gelatin or
pectin–gelatin combinations are used to attain specific textures.