아미노산 : Cysteine 시스테인
단백질 ≫ 아미노산
Cysteine 시스테인
황함유
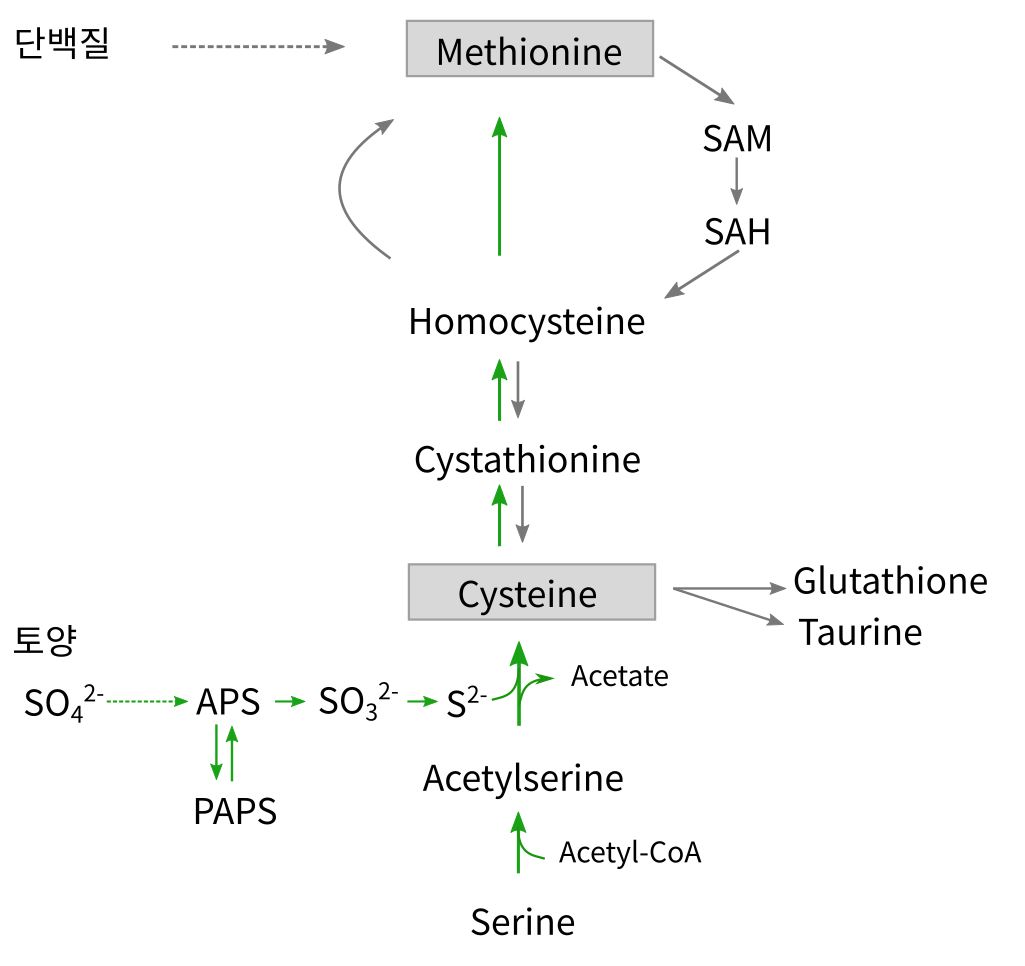
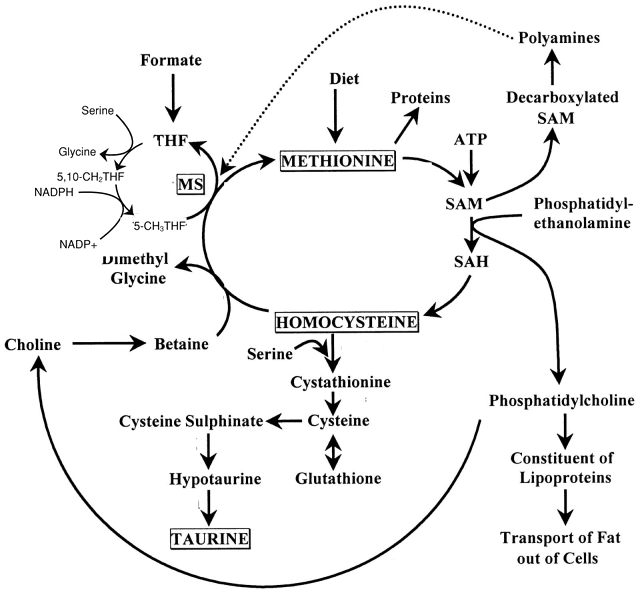
- 메치오닌 methionine
- Cysteine --> 타우린
- Glutatione : cysteine+glutamate+glycine
- 알파 리포산
머리털 ·뿔 등의 단백질의 성분으로 케라틴에는 10% 이상 함유
고등식물 효모에는 NADH(니코틴산아 미드아데닌디뉴클레오티드인산)에 의해 시스틴이 시스테인으로 되는 환원계가 존재
폴리펩티드 사슬의 고차구조의 결정과 효소, 호르몬 활성에 시스틴이 중요한 역할
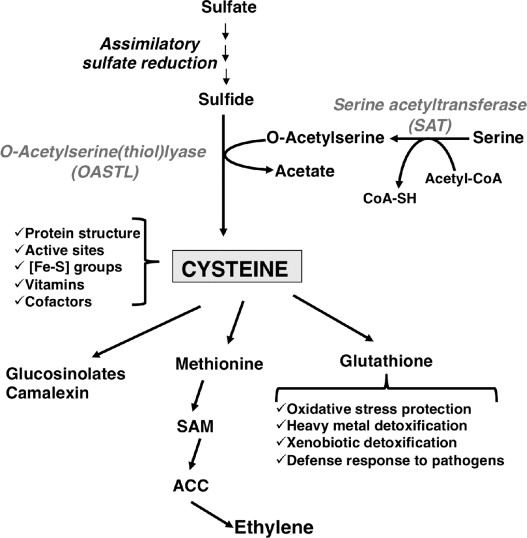
시스테인 티올 그룹은 산화환원력이 있고 반응성이 높아 수많은 생물학적인 기능이 있다. 항산화제인 글루타치온의 일부로 작동한다. 글루타치온은 시스테인, 글리신, 글루탐산이 결합하여 만들어진 분자인데 글루탐산과 글리신은 식품에 흔하지만 시스테인은 희소한 아미노산이다 있다. 철-황 클러스터의 전구체로 인간의 신진 대사에서 황화물의 중요한 원천이다
티올 그룹 덕분에 다른 금속과도 결합하는 능력도 강해 효소 중에서 시스테인을 이용하여 아연, 구리, 철과 결합한 것이 있다. 중금속과도 결합력이 강해 시스테인을 함유 한 단백질은 수은, 납 및 카드뮴과도 단단히 결합하는 문제가 있다.
시스테인은 보통 친수성 아미노산으로 간주되어왔다. 그러나 친수성은 그리 크지 않아서 비극성 아미노산과도 잘 어울린다. 시스테인 잔기의 소수성 경향은 메티오닌과 티로신과 동일하며, 세린 및 트레오닌과 같은 알려진 극성 아미노산보다 훨씬 크다. 이런 성질이 단백질 내에서 디설파이드 결합을 용이하게 하는데 영향을 준다.
시스테인의 디설파이드 결합은 단백질의 접힘과 안정성에 중요한 역할을 한다. 하지만 대부분의 세포 안에서는 환원 환경이기 때문에 디설파이드 결합이 세포질에서 불안정하다. 그리고 다른 황 함유 아미노산인 메티오닌은 디설파이드 결합을 형성 할 수 없다. 디설파이드 결합에 의한 단백질을 가교 결합은 단백질의 강도를 증가시키고 단백질 분해 저항성을 부여하여 단백질의 3차 구조를 지지한다. 인슐린은 2 개의 분리된 펩타이드 사슬이 디설파이드 결합에 의해 연결된 것이다. 이런 특성 때문에 미용(파머)에도 쓰인다. 머리카락은 단백질이고, 단백질 내에 시스테인에 환원제를 처리하면 시스테인 결합이 풀려 머리카락이 풀리고, 모양을 형성한 상태에서 디설파이드 결합을 시키면 모양이 그대로 유지된다.
시스테인의 가장 큰 응용 분야 중 하나는 풍미(반응향)의 생산이다. 시스테인과 환원당이 Maillard 반응을 하면 육류의 맛을 낸다. 또 제빵에 가공 보조제로 사용하면 물성의 개선과 풍미의 향상에 도움이 된다.
시스테인은 간 손상이나 숙취 등 알코올의 부작용에 대한 예방 또는 해독제로 제안되기도 한다. 시스테인이 아세트알데히드를 비교적 무해한 아세트산으로 바꾸는 대사 활동을 도운다. 시스테인은 의약품에서는 아미노산수액이나 간질환치료제 외에 경구, 경장영양제에 이용되고 있다. 또, 체내에서 대사되면 유황을 발생하여 다른 물질과 반응함으로써 해독작용이 있어 유해금속이나 흡연, 음주 등으로 생기는 활성산소나 방사선 등의 위해로부터 신체를 지키기 위해서 해독제나 조혈제에 사용되고 있다.
조직공학이나 바이오테크놀로지분야에서는 인프란트 등의 생체재료로 응용하기 위해 티올기의 부가 등에 따른 콜라겐의 개선을 하여 시스틴결합을 통해서 가교 가능한 콜라겐유도체가 개발되고 있다.
시스틴은 체내에서는 필수아미노산인 메치오닌으로부터 합성되지만, 체내의 메치오닌이 결핍된 경우는 시스틴의 합성이 상대적으로 줄기 때문에 케라틴의 양도 저하하여 모발이나 손톱이 약해진다. 그래서 모발이나 손톱의 질을 개선하기 위해 시스틴을 음료나 식품으로서 섭취하는 것도 효과적이지 않을까 검토되고 있다. 또, 시스틴을 케라틴과 병용해서 섭취함으로써 흡수효과 향상을 기대할 수 있다.
The cysteine thiol group is nucleophilic and easily oxidized. The reactivity is enhanced when the thiol is ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell.[9] Because of its high reactivity, the thiol group of cysteine has numerous biological functions.
- Precursor to the antioxidant glutathione
Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. Cysteine's antioxidant properties are typically expressed in the tripeptide glutathione, which occurs in humans as well as other organisms. The systemic availability of oral glutathione (GSH) is negligible; so it must be biosynthesized from its constituent amino acids, cysteine, glycine, and glutamic acid. Glutamic acid and glycine are readily available in most Western diets, but the availability of cysteine can be the limiting substrate.[citation needed]
- Disulfide bonds
Disulfide bonds play an important role in the folding and stability of some proteins, usually proteins secreted to the extracellular medium.[10] Since most cellular compartments are reducing environments, disulfide bonds are generally unstable in the cytosol with some exceptions as noted below.
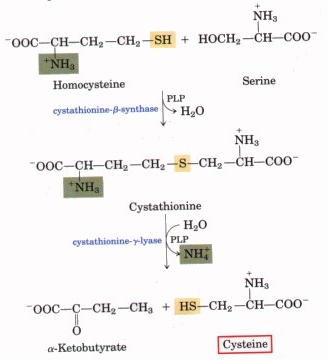
Disulfide bonds in proteins are formed by oxidation of the thiol groups of cysteine residues. The other sulfur-containing amino acid, methionine, cannot form disulfide bonds. More aggressive oxidants convert cysteine to the corresponding sulfinic acid and sulfonic acid. Cysteine residues play a valuable role by crosslinking proteins, which increases the rigidity of proteins and also functions to confer proteolytic resistance (since protein export is a costly process, minimizing its necessity is advantageous). Inside the cell, disulfide bridges between cysteine residues within a polypeptide support the protein's tertiary structure. Insulin is an example of a protein with cystine crosslinking, wherein two separate peptide chains are connected by a pair of disulfide bonds.
Protein disulfide isomerases catalyze the proper formation of disulfide bonds; the cell transfers dehydroascorbic acid to the endoplasmic reticulum, which oxidises the environment. In this environment, cysteines are, in general, oxidized to cystine and are no longer functional as a nucleophiles.
- Precursor to iron-sulfur clusters
Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.[11]
- Metal ion binding
Beyond the iron-sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues. Examples include zinc in zinc fingers and alcohol dehydrogenase, copper in the blue copper proteins, iron in cytochrome P450, and nickel in the [NiFe]-hydrogenases.[12] The thiol group also has a high affinity for heavy metals, so that proteins containing cysteine, such as metallothionein, will bind metals such as mercury, lead, and cadmium tightly.[13]
- Post-translational modifications
Aside from its oxidation to cystine, cysteine participates in numerous posttranslational modifications. The nucleophilic thiol group allows cysteine to conjugate to other groups, e.g., in prenylation. Ubiquitin ligases transfer ubiquitin to its pendant, proteins, and caspases, which engage in proteolysis in the apoptotic cycle. Inteins often function with the help of a catalytic cysteine. These roles are typically limited to the intracellular milieu, where the environment is reducing, and cysteine is not oxidized to cystine.
- Applications
Cysteine, mainly the L-enantiomer, is a precursor in the food, pharmaceutical, and personal care industries. One of the largest applications is the production of flavors. For example, the reaction of cysteine with sugars in a Maillard reaction yields meat flavors.[14] L-cysteine is also used as a processing aid for baking.[15]
In the field of personal care, cysteine is used for permanent wave applications predominantly in Asia. Again the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides will selectively attach to cysteine using a covalent Michael addition. Site-directed spin labeling for EPR or paramagnetic relaxation enhanced NMR also uses cysteine extensively.
In a 1994 report released by five top cigarette companies, cysteine is one of the 599 additives to cigarettes. Like most cigarette additives, however, its use or purpose is unknown.[16] Its inclusion in cigarettes could offer two benefits: Acting as an expectorant, since smoking increases mucus production in the lungs; and increasing the beneficial antioxidant glutathione (which is diminished in smokers).
- Sheep
Cysteine is required by sheep in order to produce wool: It is an essential amino acid that must be taken in as food from grass. As a consequence, during drought conditions, sheep stop producing wool; however, transgenic sheep that can make their own cysteine have been developed.[17]
- Reducing toxic effects of alcohol
Cysteine has been proposed as a preventative or antidote for some of the negative effects of alcohol, including liver damage and hangover. It counteracts the poisonous effects of acetaldehyde, which is the major by-product of alcohol metabolism and is responsible for most of the negative aftereffects and long-term damage associated with alcohol use (but not the immediate effects of drunkenness). Cysteine supports the next step in metabolism, which turns acetaldehyde into the relatively harmless acetic acid. In a rat study, test animals received an LD50 dose of acetaldehyde (the amount that normally kills half of all animals). Those that received cysteine had an 80% survival rate; when both cysteine and thiamine were administered, all animals survived.[18] There is not yet direct evidence for or against its effectiveness in humans who consume alcohol at normal levels.
- N-acetylcysteine (NAC)
N-acetyl-L-cysteine (NAC) is a derivative of cysteine wherein an acetyl group is attached to the nitrogen atom. This compound is sold as a dietary supplement commonly claiming antioxidant and liver-protecting effects. NAC is often used as a cough medicine because it breaks up the disulfide bonds in the mucus and thus liquefies it, making it easier to cough up. It is also this action of breaking disulfide bonds that makes it useful in thinning the abnormally thick mucus in Cystic Fibrosis patients. NAC is also used as a specific antidote in cases of acetaminophen overdose.

|
|
Network ≫ 두뇌, 마음, 욕구
뇌의 작동원리, 지도원리
감각 기관
보상시스템
- 학습 파페츠
- 목적 동기 부여 회로
식욕 mechanism
- Food Pleasure
file.txt
file.txt
|