아질산 N02 의 역할
첨가물 ≫ 발색,표백 ≫ 아질산
아질산 N02 의 역할
아질산의 생리적 기능
- 식물 방어기작의 신호
- 신경전달물질 : 신경전달 Gas
- 혈관 팽창제 : 혈압강화, vasodilator, bronchodilator
- 협심증 치료제, 비아그라, 정력제
- 침에서 유해균 억제
- 아질산 엄마의 선물 : 면역력 강화
- 청산가리(-C≡N)의 독성을 해독하는 기능을 한다
- 채소의 질산, 아질산 작용
식품에서 역할
- 헤모글로빈 산화(변색) 억제
- 식중독 예방 : 보톡스균 억제
- 풍미의 증진
NO is an important regulator and mediator of numerous processes in the nervous, immune and cardiovascular systems , including smooth muscle relaxation thus resulting in vasodilation of the artery and increasing blood flow, neurotransmission in the nervous system and has been associated with neuronal activity and various functions like avoidance learning, macrophage mediated cytotoxicity for microbes and tumor cells. Besides mediating normal functions, NO has been implicated in pathophysiologic states as diverse as septic shock, hypertension, stroke, and neurodegenerative diseases. [2] Currently, exogenous NO sources constitute a powerful way to supplement NO when the body cannot generate enough for normal biological functions. So, recent developments of novel NO donors, NO releasing devices as well as innovative improvements to current NO donors have been investigated. [3]
무기질산염 : 과일과 채소가 심혈관질환을 막고 당뇨병을 예방하는 성분
3일 스웨덴 캐롤린스카연구소 연구팀이 `세포대사학저널`에 밝힌 연구결과에 의하면 소량의 무기질산염을 3일동안 섭취한 후 건강한 사람들이 자전거를 타거나 운동을 하는 동안 산소를 덜 소모하는 것으로 나타났다. 연구팀은 "이번 연구결과만으로 무기질산염 보충제를 섭취할 필요는 없으며 이번 연구를 통해 과일과 채소 특히 잎이 많은 녹색 채소를 섭취하는 것이 건강에 왜 좋은지에 대한 설명을 한 가지 더 할 수 있게 됐다"라고 밝혔다. 연구팀은 "시금치 한 접시 혹은 붉은 근대 2-3개에 함유된 정도의 질산염을 섭취하는 정도면 이 같은 건강의 이로움을 얻을 수 있다"라고 밝혔다. 연구팀은 "과일과 채소가 심혈관질환을 막고 당뇨병을 예방한다는 것은 그 동안 잘 알려져 왔지만 과연 어떤 활성성분이 이 같은 효과를 내는지는 불확실했던 바 이번 연구결과 무기질산염이 중요한 역할을 하는 것으로 밝혀졌다"라고 강조했다.
Induction
Platelet derived factors, shear stress, acetylcholine, and cytokines stimulate the production of NO by endothelial nitric oxide synthase (eNOS). eNOS synthesizes NO from the terminal guanidine-nitrogen of L-arginine and oxygen and yields citrulline as a byproduct. NO production by eNOS is dependent on calcium-calmodulin and other cofactors.
Phosphorylation
NO, a highly reactive free radical, then diffuses into the smooth muscle cells of the blood vessel and interacts with soluble guanylate cyclase. Nitric oxide stimulates the soluble guanylate cyclase to generate the second messenger cyclic GMP (3’,5’ guanosine monophosphate) from guanosine triphosphate (GTP). The soluble cGMP activates cyclic nucleotide dependent protein kinase G (PKG or cGKI). PKG is a kinase that phosphorylates a number of proteins that regulate calcium concentrations, calcium sensitization, hyperpolarize cell through potassium channels, actin filament and myosin dynamic alterations that result in smooth muscle relaxation. (see smooth muscle article).[5]
Neurotransmission
Nitric oxide also serves as a neurotransmitter between nerve cells, part of its general role in redox signaling. Unlike most other neurotransmitters that only transmit information from a presynaptic to a postsynaptic neuron, the small, uncharged, and fat-soluble nitric oxide molecule can diffuse widely and readily enters cells. Thus, it can act on several nearby neurons, even on those not connected by a synapse. At the same time, the short half-life of NO means that such action will be restricted to a limited area, without the necessity for enzymatic breakdown or cellular reuptake. NO is also highly reactive with other free radicals, lipids, and proteins.
NO-cGMP cascade is involved in learning and memory through the maintenance of long-term potentiation (LTP).[6][7]
Nitric oxide is an important non-adrenergic, non-cholinergic (NANC) neurotransmitter in various parts of the gastrointestinal tract. It causes relaxation of the gastrointestinal smooth muscle. In the stomach it increases the capacity of the fundus to store food/fluids.
Dietary nitrate is also an important source of nitric oxide in mammals. Green, leafy vegetables and some root vegetables (such as beetroot) have high concentrations of nitrate. When eaten and absorbed into the bloodstream nitrate is concentrated in saliva (about 10 fold) and is reduced to nitrite on the surface of the tongue by a biofilm of commensal facultative anaerobic bacteria. This nitrite is swallowed and reacts with acid and reducing substances in the stomach (such as ascorbate) to produce high concentrations of nitric oxide. The purpose of this mechanism to create NO is thought to be both sterilization of swallowed food, to prevent food poisoning and to maintain gastric mucosal blood flow. A similar mechanism is thought to protect the skin from fungal infections, where nitrate in sweat is reduced to nitrite by skin commensal organisms and then to NO on the slightly acidic skin surface.
Other functions
Nitric oxide also acts on cardiac muscle to decrease contractility and heart rate. NO contributes to the regulation of cardiac contractility. Emerging evidence suggests that coronary artery disease (CAD) is related to defects in generation or action of NO. [8]
The bacterium Deinococcus radiodurans can withstand extreme levels of radioactivity and other stresses. In 2009 it was reported that nitric oxide plays an important role in the bacteria's recovery from radiation exposure: the gas is required for division and proliferation after DNA damage has been repaired. A gene was described that increases nitric oxide production after UV radiation, and in the absence of this gene the bacteria were still able to repair DNA damage but wouldn't grow.[9]
Pathology
People with diabetes usually have lower levels of Nitric Oxide than patients without diabetes[10]. Diminished supply of Nitric Oxide can lead to vascular damage, such as endothelial dysfunction and vascular inflammation. Vascular damage can lead to decreased blood flow to the extremities, causing the diabetic patient to be more likely to develop Neuropathy, non-healing ulcers, and be at a greater risk for lower limb amputation.
Pharmaceutical analogs
Nitroglycerin, amyl nitrite, "poppers" (isobutyl nitrite or similar) and other nitrite derivatives are used in the treatment of heart disease: The compounds are converted to nitric oxide (by a process that is not completely understood), which in turn dilates the coronary artery (blood vessels around the heart), thereby increasing its blood supply. These drugs, however, are predominantly venodilators, dilating peripheral veins and hence reducing venous return and preload to the heart. This reduces the oxygen requirement of the myocardium and subsequently lessens the anginal pain felt with myocardial ischemia.[11]
● Nitric Oxide(NO)의 역할
NO는 대부분의 포유류 동물의 세포내에서 생성되고 신경계에서는 화학적 신호 전달 물질로서, 혈관계에서는 혈압 조절과 혈소판의 응집 및 호중성구의 집합 작용을(Feldman 등, 1993; Gally 등, 1990; Moncada 등, 1991; Kubes 등, 1991; Ou 등, 1997), 골격근에서는 대사와 근 수축 조절 등 생리학적으로 중요한 역할을 하고(Nakane 등, 1993; Kobzik 등, 1994), 표적 세포 및 숙주 세포에 여러 가지 생리학적이며 대사 적인 변화를 유도한다(Hibbs 등, 1987).
NO의 생리학적 역할은 혈관 반응에서 활발히 연구 되었으며(Busse 등, 1990; Nakayama 등, 1992), 최근에는 NO가 신경계의 생리학적 전달자로서 뿐만 아니라(Gally 등, 1990), 염증 반응(Boughton-Smith 등, 1993; Lalenti 등, 1993; Middleton 등, 1993; Nozaki 등, 1997; Flynn 등, 1998; Sasaki 등, 1998), 면역계 및 세포 독성(Ioannidis와 Groot, 1993) 외에도 세포의 분화나 세포내 신호 전달 등의 중요한 조절 물질로 알려지고 있다. 또한 NO는 패혈증성 쇼크, 고혈압, 발작 및 신경 퇴행성 질환 등에서도 많이 발견된다(Luss 등, 1996). 한편, 면역 세포에서 iNOS에 의해 생성된 NO는 다량으로 외부의 자극에 의해 유전자 수준에서 발현되고 주로 침입한 미생물이나 종양 세포에 대해 독성을 갖는 방어 물질로서 작용하는 것으로 알려져 있다(Keller 등, 1990; Luss 등, 1996; Hierholzer 등, 1998).
NO는 세포의 guanylyl cyclase의 활성 부위 내의 철과 결합하여 guanosine triphosphate(GTP)로부터 cGMP생성을 촉진하며(Ignaro 등, 1987; Inoue 등, 1998), NO는 또한 ADP-ribosylation 효소인 glyceraldehyde-3-phosphate dehydrogenase에 영향을 미치고(Dimmeler 등, 1992), superoxide와 반응하여 peroxynitrite를 형성하여 신경세포 독성을 조절할 수 있다(Beckman과 Koppenol, 1996)(Fig. 3). 최근에 NO가 세포 활성화 물질 및 reactive oxygen intermediates(ROI)에 의해 유발된 세포 독성을 최소화 시키는 역할이 밝혀짐으로 이 분야에 대해서 활발히 연구되고 있다(Hibbs 등, 1990; Wink 등, 1996; Kr?ncke 등, 1997).
● 간에서 Nitric Oxide(NO)와 Superoxide
간은 혈관을 구성하는 내피 세포, hepatocyte(HC) 및 대식 세포인 Kupffer cell (KC) 등으로 구성되어 있다(Fig. 5). 패혈증 및 염증 상태에 있는 간에는 많은 KC와 염증 세포들이 모여들어 활성화되고 인접한 HC의 기능을 조절하는 cytokine들을 분비한다. KC에서 분비된 interleukin-6(IL-6)나 interleukin-1(IL-1)과 같은 cytokine들은 HC의 급성기 반응 물질 합성을 유도함으로서 염증 반응에 의해서 손상된 간 기능을 개선한다(Geller 등, 1993). 또한 HC 및 KC는 세포 활성 물질인 tumor necrosis factor-α(TNF-α), IL-1 및 interferon-γ(IFN-γ) 등의 혼합 처리에 의해서 활성화되어 다량의 NO를 생성한다(Harbchet 등, 1994). NO는 빠르게 산화되어 NO 2-/NO3-로 전환되며 NO를 포함한 그 산화 물질들을 reactive nitrogen intermediates(RNI)라고 부른다. 한편 염증 상태에서 KC는 NO 외에도 다량의 superoxide를 생성시켜 면역 작용과 동시에 심한 간 손상을 유발한다(Nakazawa 등, 1996; Jourd'heuil 등, 1997). Superoxide의 생성은 세포내 NADP oxidase에 의하여 생성되며(Fig. 1.B), 이 superoxide는 superoxide dismutase(SOD)에 의하여 hydrogen peroxide(H2O2)로 전환되고 H2 O2는 catalase(CAT)에 의해서 무해한 H2O로 된다. 그러나, 다량으로 생성된 H2O2 는 철 이온을 촉매로 하여 hydroxyl radical (.OH)과 같은 독성 물질을 형성한다(Miles 등, 1996). 이러한 독성 물질을 ROI라고 하며 이들의 독성을 감소시키는 물질이 antioxidant이다.
간에서 생성된 NO는 간 세포의 총단백질 합성 및 mitochondrial aconitase activity를 감소시키고, iNOS 발현은 급성기 반응 물질로부터 조절된다고 알려져 있으며(Geller 등, 1994; Kueose 등, 1996), NO가 endotoxin이나 세포 활성 물질 및 ROI에 의해 유발된 세포 독성을 최소화 시키는 역할을 하고 있음이 밝혀짐으로 이 분야에 대해서 활발히 연구되고 있으나(Billiar 등, 1990; Kr?ncke 등, 1997), NO의 생성 기전은 잘 알려져 있지 않다.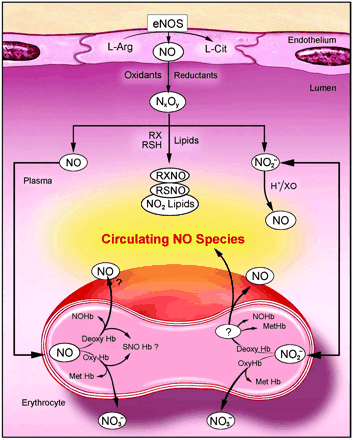
|
|
Network ≫ 두뇌, 마음, 욕구
뇌의 작동원리, 지도원리
감각 기관
보상시스템
- 학습 파페츠
- 목적 동기 부여 회로
식욕 mechanism
- Food Pleasure
file.txt
file.txt
|