| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
암 ≫ 오해 ≫ 발암물질 메탄올 : methanol 포르말린, 포름알데히드(HCHO) - 포르말린 통조림 사건 - 포르말린 우유 사건 - 생명의 원료 : 포름알데히드 -> 리보스 -> Adenosine - 메탄올의 생성 Methanol May Function as a Cross-Kingdom Signal Yuri L. Dorokhov ... Published: April 26, 2012 https://doi.org/10.1371/journal.pone.0036122 Recently, we demonstrated that leaf wounding results in the synthesis of pectin methylesterase (PME), which causes the plant to release methanol into the air. Methanol emitted by a wounded plant increases the accumulation of methanol-inducible gene mRNA and enhances antibacterial resistance as well as cell-to-cell communication, which facilitates virus spreading in neighboring plants. We concluded that methanol is a signaling molecule involved in within-plant and plant-to-plant communication. Methanol is considered to be a poison in humans because of the alcohol dehydrogenase (ADH)-mediated conversion of methanol into toxic formaldehyde. However, recent data showed that methanol is a natural compound in normal, healthy humans. These data call into question whether human methanol is a metabolic waste product or whether methanol has specific function in humans. Here, to reveal human methanol-responsive genes (MRGs), we used suppression subtractive hybridization cDNA libraries of HeLa cells lacking ADH and exposed to methanol. This design allowed us to exclude genes involved in formaldehyde and formic acid detoxification from our analysis. We identified MRGs and revealed a correlation between increases in methanol content in the plasma and changes in human leukocyte MRG mRNA levels after fresh salad consumption by volunteers. Subsequently, we showed that the methanol generated by the pectin/PME complex in the gastrointestinal tract of mice induces the up- and downregulation of brain MRG mRNA. We used an adapted Y-maze to measure the locomotor behavior of the mice while breathing wounded plant vapors in two-choice assays. We showed that mice prefer the odor of methanol to other plant volatiles and that methanol changed MRG mRNA accumulation in the mouse brain. 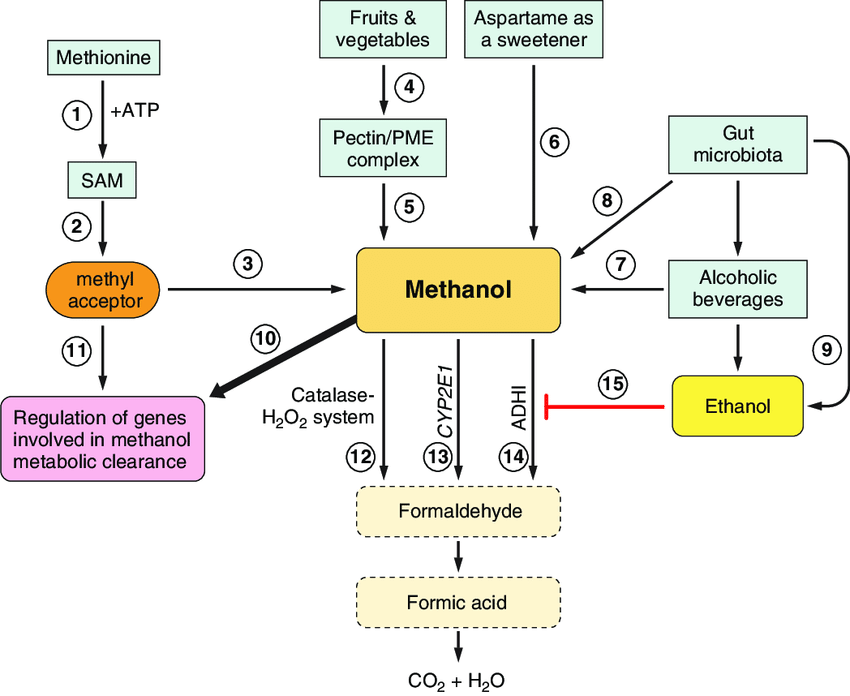 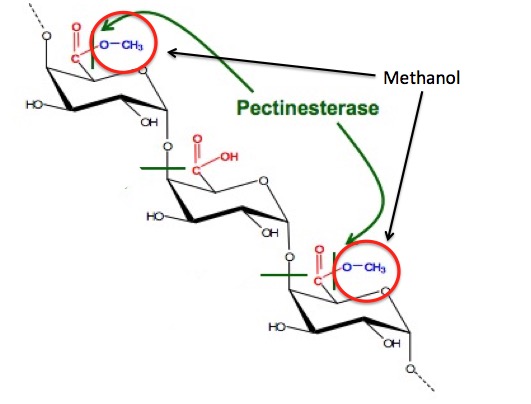 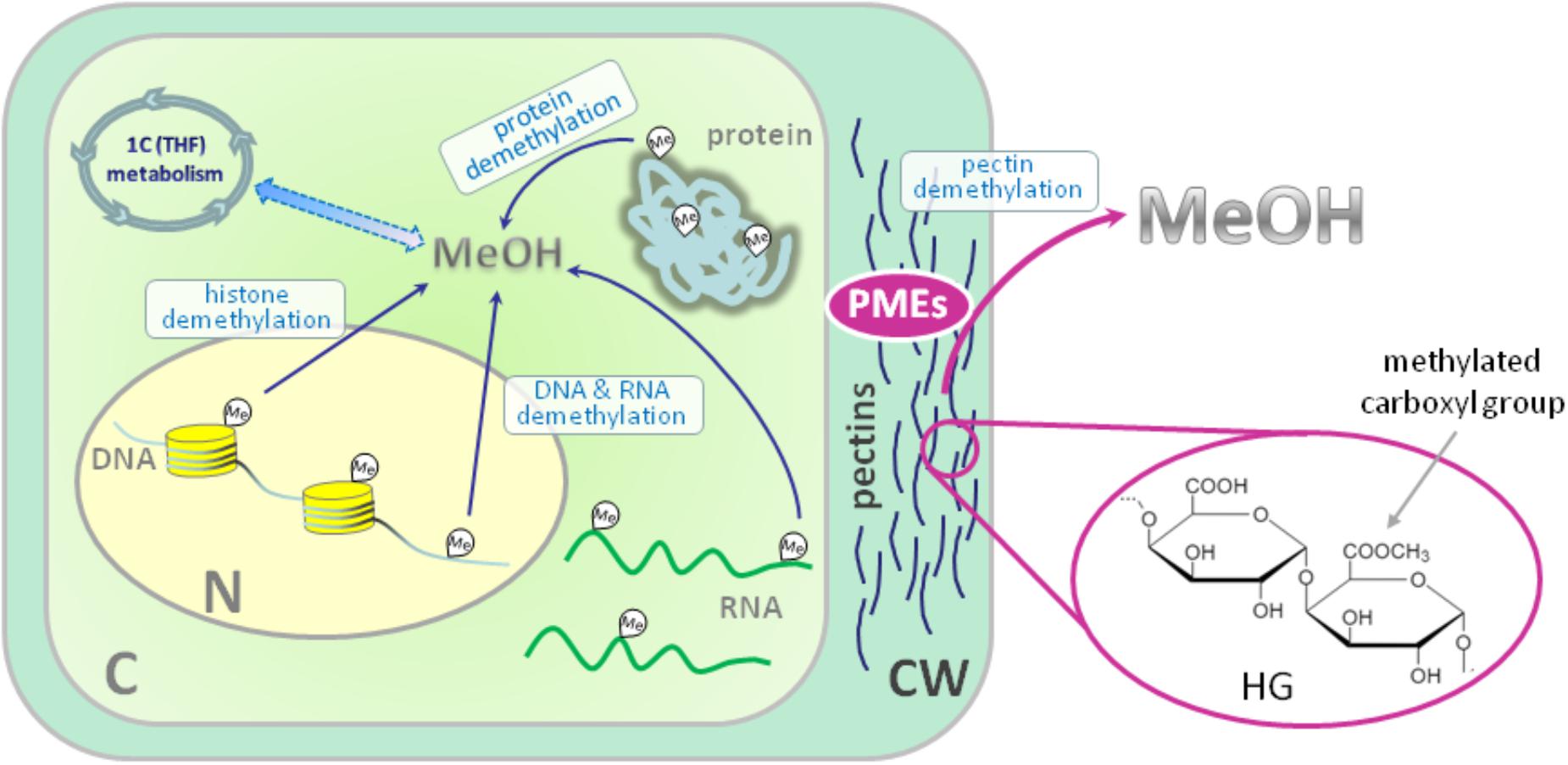 Until recently, plant-emitted methanol was considered a biochemical by-product, but studies in the last decade have revealed its role as a signal molecule in plant-plant and plant-animal communication. Moreover, methanol participates in metabolic biochemical processes during growth and development. The purpose of this review is to determine the impact of methanol on the growth and immunity of plants. Plants generate methanol in the reaction of the demethylation of macromolecules including DNA and proteins, but the main source of plant-derived methanol is cell wall pectins, which are demethylesterified by pectin methylesterases (PMEs). Methanol emissions increase in response to mechanical wounding or other stresses due to damage of the cell wall, which is the main source of methanol production. Gaseous methanol from the wounded plant induces defense reactions in intact leaves of the same and neighboring plants, activating so-called methanol-inducible genes (MIGs) that regulate plant resistance to biotic and abiotic factors. Since PMEs are the key enzymes in methanol production, their expression increases in response to wounding, but after elimination of the stress factor effects, the plant cell should return to the original state. The amount of functional PMEs in the cell is strictly regulated at both the gene and protein levels. There is negative feedback between one of the MIGs, aldose epimerase-like protein, and PME gene transcription; moreover, the enzymatic activity of PMEs is modulated and controlled by PME inhibitors (PMEIs), which are also induced in response to pathogenic attack. 최근까지 식물 방출 메탄올은 생화학 부산물로 여겨졌지만 지난 10 년간의 연구에서 식물 식물 및 식물-동물 통신에서 신호 분자로서의 역할이 밝혀졌습니다. 또한 메탄올은 성장과 발달 과정에서 대사 생화학 적 과정에 참여합니다. 이 검토의 목적은 메탄올이 식물의 성장과 면역에 미치는 영향을 결정하는 것입니다. 식물은 DNA 및 단백질을 포함하는 거대 분자의 탈 메틸화 반응에서 메탄올을 생성하지만, 식물 유래 메탄올의 주요 공급원은 세포벽 펙틴이며, 이는 펙틴 메틸 에스 테라 제 (PME)에 의해 탈 메틸 에스테르 화된다. 메탄올 생산의 주요 원천 인 세포벽의 손상으로 인한 기계적 상처 또는 다른 스트레스에 대한 반응으로 메탄올 배출량이 증가합니다. 상처 입은 식물로부터의 가스 메탄올은 동일하고 인접한 식물의 온전한 잎에서 방어 반응을 유도하여, 식물성 및 비 생물 적 요인에 대한 식물 저항성을 조절하는 소위 메탄올 유도 유전자 (MIG)를 활성화시킨다. PME는 메탄올 생산의 주요 효소이기 때문에 상처에 대한 반응으로 발현이 증가하지만 스트레스 요인 영향을 제거한 후에는 식물 세포가 원래 상태로 돌아와야합니다. 세포 내 기능성 PME의 양은 유전자 및 단백질 수준 모두에서 엄격하게 조절된다. MIG, 알 도스 에피 머라 제-유사 단백질 및 PME 유전자 전사 중 하나 사이에 음성 피드백이 있으며; 또한, PME의 효소 활성은 병원성 공격에 대한 반응으로 유도되는 PME 억제제 (PMEI)에 의해 조절되고 제어된다. Methanol Participation in Plant Growth and Development The participation of methanol in growth and development is determined by the function of pectins in the formation of the cell wall. Pectin polysaccharides are synthesized in the cis-, medial- and trans-Golgi (Sandhu et al., 2009; Atmodjo et al., 2013; Kim and Brandizzi, 2014, 2016). Numerous enzymes of pectin biosynthesis located in the Golgi apparatus were isolated and described, and their transmembrane type II topology assuming absence of a cleavable signal sequence was proved (Atmodjo et al., 2013). Methyl esterification of pectins probably occurs simultaneously with its synthesis (Atmodjo et al., 2013). With the help of secreted vesicles moving mainly along the actin cytoskeleton, pectins are delivered in a highly methylesterified form to the cell wall, which is building up at the stage of formation of the cell plate separating two new cells (Daher and Braybrook, 2015). The role of pectins in plant growth and development is evidenced by the phenotypes of Arabidopsis mutants and transgenic lines, in which the synthesis and modification of individual pectin polysaccharides is disrupted (Atmodjo et al., 2013; Saffer, 2018). Plants with altered HG length also have multiple developmental defects, including an increased number of petals, variable phyllotaxis and early flowering, indicating that HG mechanical properties affect different developmental processes (Xiao et al., 2014, 2017). Mutants that affect pectin synthesis suffer from dwarfism and have decreased cell lengthening (Krupková et al., 2007; Saffer et al., 2017). Methyl esterification of HG is a key determinant of cell wall properties, growth and leaf position (Peaucelle et al., 2008, 2012; Müller et al., 2013; Daher and Braybrook, 2015; Levesque-Tremblay et al., 2015; Hocq et al., 2017). Since most HG is synthesized in the methyl esterified form, during the growth of the cells it can be demethylesterified by the action of the PME enzymes, which are in turn regulated by a pectin methylesterase inhibitors (PMEIs) (Wolf et al., 2009; Jolie et al., 2010). After demethylesterification, HG can form Ca2+-pectate-cross-linked complexes, referred to as “eggboxes,” which strengthen the cell wall (Peaucelle et al., 2012; Daher and Braybrook, 2015). This process is observed, for example, during Nicotiana tabacum pollen tubes growth (Holdaway-Clarke et al., 2003; Bosch et al., 2005). On the other hand, increased PME activity correlates with the accumulation of demethylesterified HG in the Arabidopsis hypocotyl and apical meristem and leads to the cell wall loosening essential for growth symmetry breaking (Peaucelle et al., 2008, 2011, 2015; Figure 2A). Pectin methylesterases interact with PMEIs to influence fruit development and ripening (Reca et al., 2012). Moreover, there are correlations between PME activity, the level of pectin de-esterification and L-ascorbic acid production in the later stages of tomato fruit ripening (Rigano et al., 2018). The participation of PMEs in the demethylesterification of HG inevitably leads to the formation of methanol. As a consequence, methanol emissions from plant leaves are much higher when the leaves are young and expanding than when they reach maturity (Nemecek-Marshall et al., 1995; Galbally and Kirstine, 2002; Oikawa et al., 2011). This phenomenon can also be observed in developing tobacco leaves at the stage of their transition from the state of acceptors (sink-leaves) of photoassimilates to the state of donors (source-leaves) (Burch-Smith and Zambryski, 2012). A similar sink-source modification occurs with the participation of tobacco PME producing methanol from pectins (Komarova et al., 2014a). When studying the role of methanol in the functioning of plasmodesmata in tobacco sink-leaves, an increased content of PME (EMBL Accession #AJ401158) transcripts (Dorokhov et al., 2006) and raised concentration of methanol in the sap and tissues of immature leaves was found. Moreover, the genes for sieve element occlusion protein (SEOP), auxin-repressed protein (ARP), salicylic acid binding catalase (SABC) involved in tobacco plant growth and signal transmission proved to be methanol-sensitive (Komarova et al., 2014a). Methanol, formed during the demethylesterification of HG, appears to also have a beneficial effect on the overall cellular metabolism. It is known that spraying plant leaves with 10–50% methanol can stimulate the photosynthetic activity and overall productivity of C3-plants (Nonomura and Benson, 1992). Although the mechanism responsible for this phenomenon is uncertain, it is assumed that formaldehyde and formic acid formed from methanol make a contribution via the folate-driven one-carbon (1C) cycle in the biosynthesis of serine, methionine, as well as purines and thymidylate in nucleic acids (Hanson and Roje, 2001). Moreover, [13C]-labeled methanol was shown to enter cells of higher plants (Acer pseudoplatanus) in suspension culture and to be metabolized to [3-13C]serine, [13CH3]methionine, and [13CH3]phosphatidylcholine in addition to the induction of the methyl-β-d-glucopyranoside de novo synthesis (Gout et al., 2000). Methanol Emission as a Stress Signal and Its Feedback Control Because the cell wall separates and protects the cell from the environment (Malinovsky et al., 2014), the PMEs contained therein play a significant role in this defense mechanism (Komarova et al., 2014b). In addition to the direct effects of PMEs on tobacco cell wall, these enzymes also act via methanol released through the demethylesterification of pectins. Mechanical damage (Dorokhov et al., 2012) and the attack of pathogens (von Dahl et al., 2006; Körner et al., 2009) increase the synthesis of tobacco PMEs, accelerate de-esterification processes and dramatically increase methanol emission (Lionetti et al., 2012; Komarova et al., 2014b; Dorokhov et al., 2015). Methanol is considered not only a by-product of PME activity but also a participant in cell signaling and an inducer of plant protection reactions (Tran et al., 2018). Transgenic tobacco plants with overexpression of the PME genes from A. thaliana or Aspergillus niger dramatically increased methanol emission and resistance to plant sap sucking pests Myzus persicae (aphid) and Bemisia tabaci (whitefly) (Dixit et al., 2013). Moreover, the released methanol can act as a signaling molecule that induces the defense responses of both the native plant and the leaves of the neighboring plant (Dorokhov et al., 2012). It has been suggested that methanol functions as a DAMP-like alarm signal, and it probably functions as an elicitor-active DAMP in monocot grasses (Hann et al., 2014). The signal function of methanol is to control the so-called MIGs that regulate plant resistance to abiotic and biotic factors (Dorokhov et al., 2012). Among the MIGs, are genes encoding aldose 1-epimerase-like protein (mutarotase) (AELP), PMEI and β-1,3-glucanase, involved in controlling the intercellular transport of macromolecules and creating favorable conditions for the reproduction of viruses but preventing bacterial colonization (Dorokhov et al., 2012, 2015; Komarova et al., 2014b). Moreover, methanol released into the air after a leaf injury results in a “priming” effect on intact leaves, setting the stage for the within-plant and neighboring plant immunity (Dorokhov et al., 2012). While the mechanism (direct or indirect) of the cellular impact of an increase of physiological methanol is unclear, it can be assumed that one of methanol′s functions is to control its own synthesis. In general, prolonged and continuous exposure to stress factors, such as prolonged mechanical wind impact, accompanied by the synthesis of PMEs, causes irreversible changes in woody plants (Hamant and Moulia, 2016) like coniferous trees on Atlantic coastal cliffs (Jaffe and Forbes, 1993). Short-term effects do not lead to the significant changes and depletion of cellular biosynthetic resources (Bostock et al., 2014), since there is a mechanism for returning the cell to its original state when the PMEs synthesis and methanol emission are downregulated after the elimination of the stress factor impact. One of the key players of such control and regulation are PMEIs. For example, during A. thaliana infection by the necrotrophic pathogen Botrytis cinerea, PME activity and pectin demethylesterification are dynamically modulated by PMEIs (Lionetti et al., 2017). Recently, another PME activity controlling mechanism was identified: the methanol-inducible gene AELP involved in intercellular transport and possibly controlling the transport and metabolism of sugars (Sheshukova et al., 2017) negatively regulates tobacco PME expression. The proposed model for the feedback regulation of gene expression involving PMEI and AELP suggests that when the cell wall is mechanically damaged, the PME genes are activated (Figure 2B); synthesis of PME and its secretion in the tobacco cell wall cause the active synthesis and emission of methanol, leading, in particular, to an increase in its concentration in the cytoplasm. This process is accompanied by induction of the PMEI genes and activation of the expression of the AELP gene through its methanol-inducible promoter. As shown in Figure 2B, AELP is capable of suppressing the transcription activity of the tobacco PME gene (Sheshukova et al., 2017) and reducing the level of methanol emission, which ultimately leads to a decrease in the transcription of the tobacco AELP gene. In accordance with this scheme, AELP proteins are likely to behave as transcription factors by inhibiting the expression of the PMEs in the nucleus and not in the apoplast. Thus, the proposed model assumes the regulation of PMEs functioning at both the gene and protein levels. |
||||
|
|
|||