| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
화학 ≫ 결합, 분해 ≫ 용해도 단백질의 용해도 : 염 농도 단백질응고 : 단백질 Unfolding - 염농도 : salt in salt out - 단백질 열응고 : 계란 열응고, 고기 열응고 - 단백질 이온 응고 : 두부 응고, - 단백질 산 응고 : 치즈 응고, 두부 GDL - 단백질 기계적 응고 : 난백 beat, 반죽 Kneading 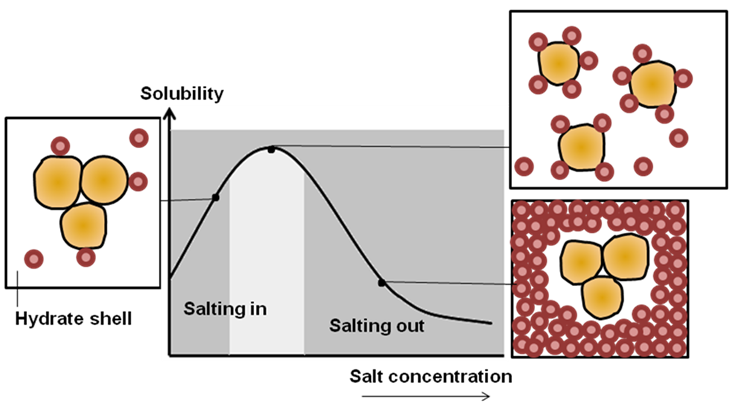 Salt in : 염은 단백질 용해도에 복잡한 영향을 미친다. 이온이 단백질 사슬간에 정전기적인 인력을 제거하면 단백질은 사슬간에 결합력이 약해서, 단백질이 따로따로 분리되어 용해도가 증가하며 물과의 결합은 증가하여 보수력이 증가한다 Salt out(염석) : 염이 고농도가 되면 단백질 중에 수분과 결합하는 극성부위에 이들 이온이 결합하여 중화되고, 단백질의 친수성이 떨어져 단백질 끼지 뭉치게 된다. Seafood proteins R. Tahergorabi, ... J. Jaczynski, in Handbook of Food Proteins, 2011 Myofibrillar proteins are soluble in concentrated saline solutions (ionic strength above 0.6) as well as extremely low ionic strength, but are water insoluble in typical physiological ionic strength in the fish muscle (ionic strength approximately 0.05 for rainbow trout). Myofibrillar proteins are composed of myosin, actin, and regulatory proteins such as tropomyosin, troponin and actinin (Fig. 6.3). Myofibrillar proteins make up 66–77% of total proteins in fish muscle and provide several functional properties that are useful in food products. Generally, seafood myofibrillar proteins are less thermally stable than the proteins isolated from terrestrial animals. The pH and ionic strength affect thermal stability of seafood myofibrillar proteins, and hence, heat-induced denaturation. Myofibrillar proteins isolated from cold water species are typically less thermally stable than warm water species. This property translates into different requirements for handling and freezing of seafood from cold and warm waters. Protein gelation and rheological properties responsible for texture development, and therefore, consumer acceptability depends mainly on the quality of myofibrillar proteins, which is affected by seafood species, age, seasonality, freshness, and processing parameters such as protein concentration, pH, ionic strength and temperature (Suzuki, 1981). MEAT Protein Y.L. Xiong, in Encyclopedia of Meat Sciences (Second Edition), 2014 Myofibrillar protein gels formed in muscle foods are generally heat induced. Two types of gels can be produced from myofibrillar proteins – the myosin gel and the mixed myofibrillar protein gel. Myosin forms filaments at ionic strengths close to the physiological condition. Hence, it can form a somewhat brittle gel at low concentrations of salt (0.15–0.20 mol l-1 NaCl, or ~0.6–0.8% in meat). Because this ionic condition is seldom employed in the process of producing ‘texturized’ meat (which requires at least 0.5 mol l-1 NaCl (~2% in meat) to ensure an adequate protein extraction and solubility, myosin gelation at the low salt condition is of little practical significance. However, mixed myofibrillar protein gels, also referred to as ‘myofibrillar protein gels’ and sometimes as ‘actomyosin gels’ or ‘salt-soluble protein gels,’ are the gels formed in most processed meats. However, even in the mixed-protein system, the myosin portion of the actomyosin remains the most important gelling protein. The contribution to the rigidity of myosin or actomyosin gels by the different segments of myosin is believed to follow the order: rod>light meromyosin>heavy meromyosin>the S-1 subunit. 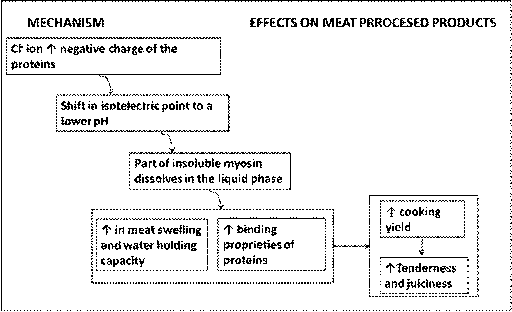  |
||||
|
|
|||