| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
Gas ≫ 이산화탄소, 산소, 질소 탄산의 맛 : 이산화탄소, CO2 농도감지 6번째 맛 : 물, 지방, 고쿠미, Ca, CO2 - 6번째 맛 : 지방 감각 - 6번째 맛 : 칼슘, kokumi - 6번째 맛 : 탄산, 이산화탄소 - 6번째 맛 : 물의 감각, 물의 맛, 물의 냄새 탄산음료의 효능 - 이산화 탄소의 농도감지 - 갈증과 온도수용체 - 온도수용체 : TRP TRP channels: sensors and transducers of gasotransmitter signals Nobuaki Takahashi1,2, Daisuke Kozai1 and Yasuo Mori1,3,4* Front. Physiol., 09 August 2012 | https://doi.org/10.3389/fphys.2012.00324 The transient receptor potential (trp) gene superfamily encodes cation channels that act as multimodal sensors for a wide variety of stimuli from outside and inside the cell. Upon sensing, they transduce electrical and Ca2+ signals via their cation channel activities. These functional features of TRP channels allow the body to react and adapt to different forms of environmental changes. Indeed, members of one class of TRP channels have emerged as sensors of gaseous messenger molecules that control various cellular processes. Nitric oxide (NO), a vasoactive gaseous molecule, regulates TRP channels directly via cysteine (Cys) S-nitrosylation or indirectly via cyclic GMP (cGMP)/protein kinase G (PKG)-dependent phosphorylation. Recent studies have revealed that changes in the availability of molecular oxygen (O2) also control the activation of TRP channels. Anoxia induced by O2-glucose deprivation and severe hypoxia (1% O2) activates TRPM7 and TRPC6, respectively, whereas TRPA1 has recently been identified as a novel sensor of hyperoxia and mild hypoxia (15% O2) in vagal and sensory neurons. TRPA1 also detects other gaseous molecules such as hydrogen sulfide (H2S) and carbon dioxide (CO2). In this review, we focus on how signaling by gaseous molecules is sensed and integrated by TRP channels. 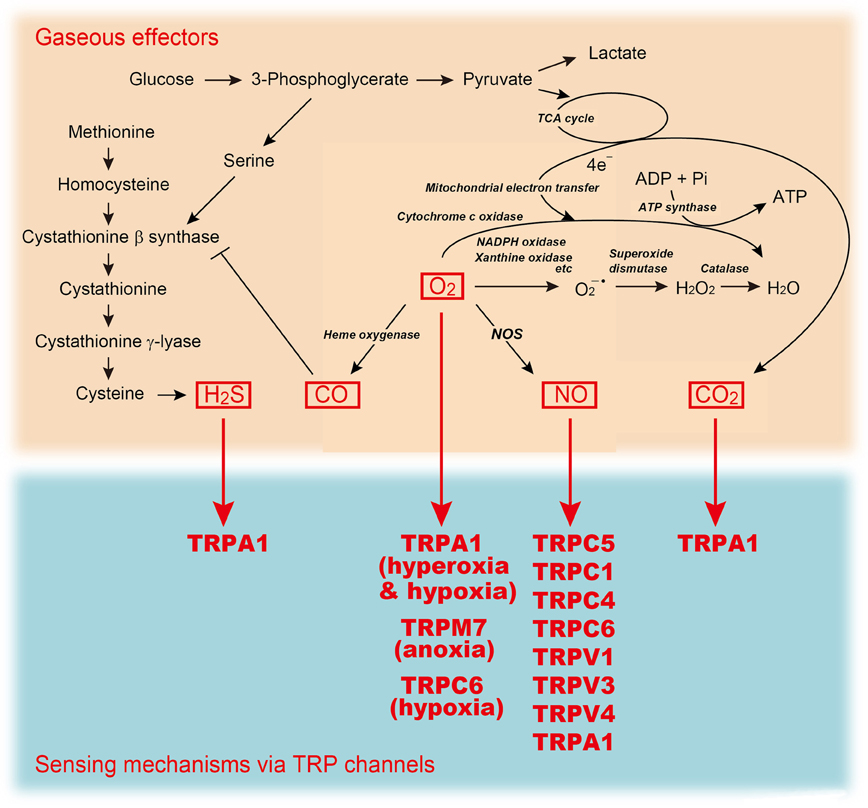 O2 Sensing Mechanisms Of the gaseous molecules, O2 is the most well-known to physicians, scientists and laymen alike as an essential physical requirement. O2 functions primarily as a terminal acceptor of electrons on mitochondrial electron transport. Most of the O2 consumed in this process is reduced to generate water through the actions of cytochrome oxidase. The remainder is used to generate compounds that exert potent biological actions, including prostaglandins, reactive oxygen species (ROS) and gaseous molecules such as NO and CO (Suematsu et al., 2003). In the last decade, O2 itself has been increasingly recognized as an important signal molecule that mediates many physiological and pathophysiological processes including proliferation of stem cells, ischemia injury and tumor progression (Csete, 2006; Swartz et al., 2007). Thus, O2 is not only required for cellular respiration, but also serves as a signaling molecule, and as the essential substrate for the formation of other signaling molecules (Figure 2). However, O2 also exerts toxicity causing aging, respiratory disorders and eventually death in a high O2 (hyperoxic) environment. Because of the ambivalent physiological nature of O2, aerobic life forms must adapt to hyperoxia and low O2 environment (hypoxia) by sensing O2 availability and transmitting this information to effector systems. Cellular responses to changes in O2 availability can be acute or chronic (López-Barneo et al., 2001). Acute responses rely mainly on O2-regulated ion channels, which mediate adaptive changes in cell excitability, contractility, and secretory activity (Gonzalez et al., 1994; Neubauer and Sunderram, 2004; Weir et al., 2005). Chronic responses depend on the modulation of transcription factors such as hypoxia-inducible factor (HIF), which determines the expression of numerous genes encoding growth factors, enzymes, and transporters (Semenza and Wang, 1992; Schofield and Ratcliffe, 2004; Webb et al., 2009). O2-regulated ion channels and transcription factors are part of a widely operating signaling system that helps to provide an appropriate amount of O2 to the tissues and to protect the cells against toxicity damage due to excess or deficient O2. In mammals, the carotid bodies, located near the carotid artery bifurcations, and brainstem catecholaminergic neurons rapidly detect changes in partial O2 pressure (PO2) in arterial blood (Gonzalez et al., 1994; Neubauer and Sunderram, 2004). It is known that BKCa, TASK, and KV K+ channels are involved in the mechanism of arterial O2 sensing in the carotid bodies (Gonzalez et al., 1994; Weir et al., 2005). Hypoxia inhibits K+ channels through several mechanisms, such as CO production by hemeoxygenase and intracellular ATP depletion that depolarizes glomus cells. This inhibition leads to activation of VDCC, exocytosis, and the excitation of carotid sinus nerves. In contrast, hyperoxia attenuates depolarization and inhibits exocytosis (Gonzalez et al., 1994; Weir et al., 2005). The chemosensory inputs of the carotid sinus nerve are carried toward the medullary centers that regulate the ventilatory pattern. The local O2 tension is also rapidly detected by other tissues including vagal and sensory neurons in the airway and lungs, chromaffin cells of the fetal adrenal medulla, smooth muscle cells of the pulmonary resistance arteries, cerebral neurons, fetoplacental arteries, systemic arteries, and the doctus arteriosus. However, detection of hypoxia by these tissues remains to be fully characterized. Recently, a class of TRP channels has been demonstrated to act as cell sensors for changes in O2 availability (Aarts et al., 2003; Weissmann et al., 2006; Takahashi et al., 2011). 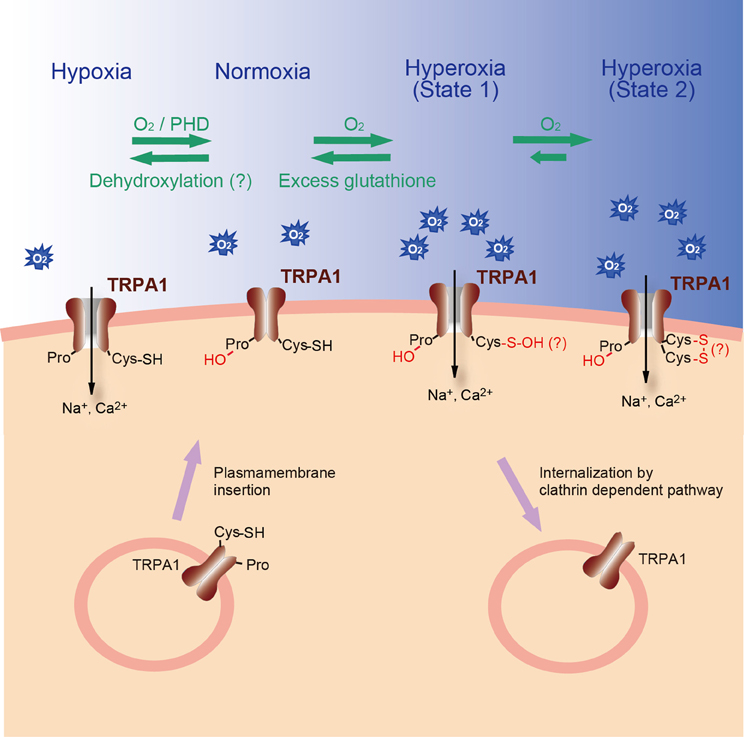 |
||||
|
|
|||