| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
제품 ≫ 커피 ≫ 향변화 커피의 쓴맛 커피의 풍미 - 커피의 향기물질 종류 : 로스팅 향 - 커피의 쓴 맛 - 쓴맛 : 쓴맛의 masking : 거의 불가 - 봄에 쓴 음식을 먹는 이유 지금까지 연구에 의해서 25~30의 물질이 커피의 쓴맛에 관여한다고 알려졌으나 2007년 까지는 어떤물질이 주 역할을 하는지 알려지지 않았다. 카페인이 주된 역할을 할 것이라 생각했지만 Thomas Hofmann 박사 (독일 뮌헨대 식품화학과 교수)의 연구에 의해 그것은 자바 커피의 15% 정도만 기여한다고 밝혀졌다. 그래서 일반 커피와 디카페인 커피의 쓴맛 차이가 그리 크지 않는 것이다. 커피는 로스팅을 강하게 하면 할수록 쓴맛이 증가한다. 따라서 로스팅 할 때 만들어진 물질 중에 주범이 있는 것이다. 호프만박사는 정밀한 분석기기와 훈련된 패널 요원의 관능검사를 통해 커피의 결정적 쓴맛이 chlorogenic acid lactones 과 phenylindanes에 의해 발생함을 밝혔다. 2가지 물질은 생두에 없는 물질로 클로로젠산으로부터 만들어진다 Chlorogenic acid lactones은 약 10가지 형태의 구조를 가지는데, 약배전~중배전한 커피에서 쓴맛을 주고 이것의 분해물인 Phenylindanes은 강배전 커피에 많다. 이것이 보다 거슬리면서 오래 남는 쓴맛을 준다. 강배전 커피가 왜 더 쓴지를 설명해 주는 물질이다. Tweaking Coffee's Flavor Chemistry Roasting, cooling, and storage conditions affect the chemicals that contribute to brew's flavor and aroma Sophie L. Rovner http://pubs.acs.org/email/cen/html/092007130322.html 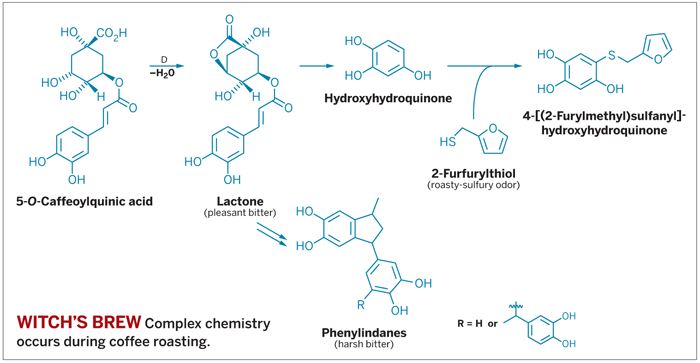 September 17, 2007 Volume 85, Number 38 pp: 32-34 ACS Meeting News COFFEE PREPARATION may appear simple, but the chemistry it touches off is complex indeed. Food scientists are studying the reactions that take place in coffee beans and in the beverage itself to make this treat even more delectable. Shutterstock The process begins when raw green coffee seeds, or beans, are removed from bright red coffee berries, then dried and roasted. Beans are typically roasted for eight to 12 minutes and ultimately reach a temperature of 210–225 °C. "The final roasting temperature influences not only the quality and quantity of aroma compounds that give coffee its enticing character but also the correct ratio between bitter and acid flavor," according to Thomas Hofmann, chair of food chemistry and molecular sensory science at the Technical University of Munich in Freising, Germany. A thousand volatile compounds have been identified in coffee, though just 40 or so of these substances "have been demonstrated to contribute to the alluring smell," Hofmann noted. They include β-damascenone (which has an aroma like cooked apples), 2-furfurylthiol (sulfury, roasty), 2-isobutyl-3-methoxypyrazine (earthy), guaiacol (spicy), 2,3-butanedione (buttery), and 4-hydroxy-2,5-dimethyl-3(2H)-furanone (caramel-like). The flavor and aroma compounds derive from multiple chemical reactions, including the Maillard reaction, caramelization, polyphenol degradation, polymerization of carbohydrates, and pyrolysis. One of the distinctive flavor characteristics of coffee is its bitterness, which develops during roasting. Conventional wisdom held that caffeine was the most important compound responsible for this flavor, Hofmann said. But he wasn't convinced, particularly when he found that consumers couldn't distinguish between decaffeinated coffee and decaf spiked with caffeine. That proved caffeine wasn't an important contributor to bitterness, Hofmann said. While caffeine is bitter, its concentration in coffee is too low to be tasted, he added. Hofmann spoke about coffee bitterness in a press briefing and symposium on thermal generation of flavors at last month's American Chemical Society national meeting in Boston. The symposium was sponsored by the Division of Agricultural & Food Chemistry. Hofmann and his research associate Oliver Frank of Germany's University of Münster turned to fractionation experiments to try to isolate the bitter factors. They found that two groups of components were significant contributors to bitterness: chlorogenic acid lactones and multiply hydroxylated phenylindanes. The lactones derive from chlorogenic acids, also referred to as O-caffeoylquinic acids, which are the predominant polyphenols present in raw coffee beans. Bean roasting either can break these phenolic acids down to form di- and trihydroxybenzenes such as hydroxyhydroquinone or can epimerize and dehydrate the acids to give various lactones that provide a "pleasant, coffeelike bitter taste quality" in light- to medium-roast coffee, Hofmann said. If roasting continues, the lactones break down and form 4-vinylcatechol as a highly reactive intermediate. This compound then oligomerizes to give multiply hydroxylated phenylindanes such as the dimeric cis- and trans-5,6-dihydroxy- 1-methyl-3-(3′,4′-dihydroxyphenyl)indanes. These compounds yield a "lingering, harsh type of bitter sensation" typical of overroasted coffee, Hofmann noted. The 20 lactones and indanes that Hofmann identified could serve as reliable markers for industrial roasters. They could use liquid chromatography coupled to mass spectrometry to track these compounds and thereby tailor roasting time and temperature to achieve an even more desirable coffee taste. This science-driven technique would be more objective than the current method, which mainly assesses the color of roasting beans to determine when roasting should be stopped. ONCE COFFEE BEANS are roasted, they stay fresh for several months, but ground coffee is best used within two weeks of grinding, according to Hofmann. Brewed coffee also evolves, with the flavor changing dramatically within a few minutes of brewing and continuing to deteriorate until the beverage is consumed. "The longer you keep coffee hot, the more you degrade the aroma molecules, and in particular, those exhibiting the pleasant sulfury-roasty smell," Hofmann said. "The acidity increases too, because the lactones are hydrolyzed to form free acids." Fresh coffee has a pH between 5.0 and 5.4; that can drop to 4.6 if the coffee is kept hot for two or three hours, increasing sourness and also influencing the perceived bitterness. Aroma degradation can be a particular problem when coffee is stored as a canned or bottled beverage or as a liquid coffee concentrate, noted Christoph Müller, a senior scientist at Firmenich in Plainsboro, N.J. He spoke in the flavor generation symposium and press briefing at the national meeting. "Unfortunately, the pleasant fresh-coffee aroma cannot be simply preserved," Müller said. Once again, it's the sulfury-roasty aroma quality that suffers during storage of coffee beverages. "This is mainly due to the decrease of the coffeelike-smelling compound 2-furfurylthiol (FFT)." Müller, who was a doctoral student with Hofmann when he performed the work he described at the meeting, wanted to find a way to prevent this "aroma staling" and extend coffee's shelf life. Müller found out that di- and trihydroxybenzenes, and in particular hydroxyhydroquinone, react with FFT and other odor-active thiols such as methanethiol that are present in freshly brewed coffee. The reactions form conjugates, including 4-[(2-furylmethyl)sulfanyl]hydroxyhydroquinone. This oxidative coupling begins right after coffee is brewed and continues when the beverage is kept hot or processed into canned, instant, or concentrated liquid coffee. The binding reactions, which end when all the odor-active thiols are trapped, explain why the smell of the stored beverage isn't as appealing as the alluring aroma of freshly brewed coffee. Shelf life could be improved by treating coffee with enzymes that would break down thiol-binding compounds such as hydroxyhydroquinone, Hofmann said. Alternatively, trapping of the thiols could be limited by tailoring the bean roasting process to reduce formation of hydroxyhydroquinone, lowering the oxygen level in coffee, or controlling parameters such as pH during extraction of coffee powder, Müller said. Jürg Baggenstoss, a Ph.D. student at the Swiss Federal Institute of Technology, Zurich, also discussed the influence of roasting conditions on flavor during the national meeting. Baggenstoss investigated the impact of time, temperature, heat transfer, and bean cooling methods on flavor formation, oxidative stability, and shelf life of coffee. He compared the traditional roasting process with a slow, low-temperature roasting process (660 seconds, 228 °C) and a fast, high-temperature process (170 seconds, 260 °C). Baggenstoss' experimental methods resulted in the same degree of roast as gauged by bean color. But the fast, high-temperature process formed considerably more aldehydes and diketones as well as 4-vinylguaiacol, N-methylpyrrole, and 3-mercapto-3-methylbutyl formate. THE RESULTS "show that different time-temperature profiles lead to different flavor profiles," Baggenstoss concluded. "The concept of a degree of roast derived from roast color can be a bit misleading." His most important finding, he added, is that "you cannot simply speed up the slow, traditional roasting process" and still attain the most pleasing flavors. Once beans reach the desired degree of roast, they are cooled rapidly with air or water. Air-cooled coffee beans contain just 1–2% water, while water-cooled coffee beans contain as much as 5% water. Baggenstoss studied the effect of the beans' water content on the stability of flavor compounds during storage. He found that aldehydes, pyrazines, and diketones such as 2,3-butanedione were unaffected by bean water content. On the other hand, compounds such as dimethyl trisulfide formed faster and reached higher levels in beans with higher water contents. Dimethyl trisulfide is formed by the oxidation of methanethiol, which is broadly related to the perception of coffee freshness. "Therefore, the coffee with higher water content seemed to lose fresh attributes faster than air-quenched coffee," Baggenstoss said. Furthermore, "some of the impact compounds are more rapidly degraded during storage of coffees with higher moisture content." Since several flavor-impact compounds were affected by water content, "we have to assume that increased water content has a negative influence on flavor stability," Baggenstoss said. "In order to increase shelf life, the final water content of the roasted coffee bean should be low." Once applied, such findings may ensure that coffee remains good to the last drop. |
||||
|
|
|||