| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
내몸 ≫ 감각 ≫ 후각 후각 : 역치 후각의 생리적 특징 - 후각 피로현상 - 역치 : 감각의 한계, 훈련에 의한 분별력 향상 - 후각 : 나이에 따른 후각의 변화 - 후각 : 남녀간 차이 - 후각 : 개인, 인종간 취향의 차이 - 후각 장애 - 페로몬 - 작은 분자는 천방지축 역치 Threshold - 미각 역치 - 후각 역치 감각역치 (Threshold of sensation) : - 자극역치 또는 절대역치로 감각이 없는 상태에서 감지가 일어나는 변화가 일어나는 강도 차별역치 (Difference of threshold) : - 주어진 자극이 감각에 대한 확실한 변화를 부여하는데 필요한 최소 변화량 한계역치 (Terminal threshold) : - 양이 증가시켜도 적절한 강도를 느낄 수 없는 량, 이 양을 초과하면 통상적으로 고통으로 느껴진다 Old: – OTV is the concentration of a compound where 50% of the population can differentiate the odour from neutral air – Population is often confused with ‘our odour panel’ – Children and older people almost always excluded • New (since 1995, Dutch standard NVN2820 and 2003 EU standard EN13725 – A traceable unit, linked to the unit Mass in SI system – Linked to an agreed reference value for a standard olfactory stimulus: 1 European Reference Odor Mass is 0.123μg n-butanol – Evaporated into 1 m3 of neutral air, 1 EROM produces, by definition, an odour concentration of 1 ouE·m-3 – EN13725 revision (2019?) includes a procedure for direct sensory linking of EROM values of different compounds, to the primary EROM for n-butanol In 1995 the technical committee TC264 air quality of the European Committee for Standardization convened the working group 2, which developed an odor testing standard. It was released in 2003 as EN 13725 air quality – measurement of odor concentration using dynamic olfactometry. As the methodology of dynamic olfactometry according this European Standard is approved among others in Australia as AS 4323.1 and in Chile as NCh 3190, the EN 13725 today is the most applied standard worldwide. Other used methods for determination of odor thresholds are ASTM 679 as well as Japanese and Chinese standards. Compared with the EN 13725 these standards are working on a lower detail level concerning technical requirements and execution description. In 2012 the TC264 took a resolution to review the standard. A revised draft standard is expected to become available in 2017. The revised standard will contain comprehensive guidance on reference material, measurement uncertainty, health and safety issues, materials for olfactometry, sample storage and the sampling of different odor sources. Once the endpoint of the revision has been reached a release of an ISO standard based on EN 13725 is envisaged. 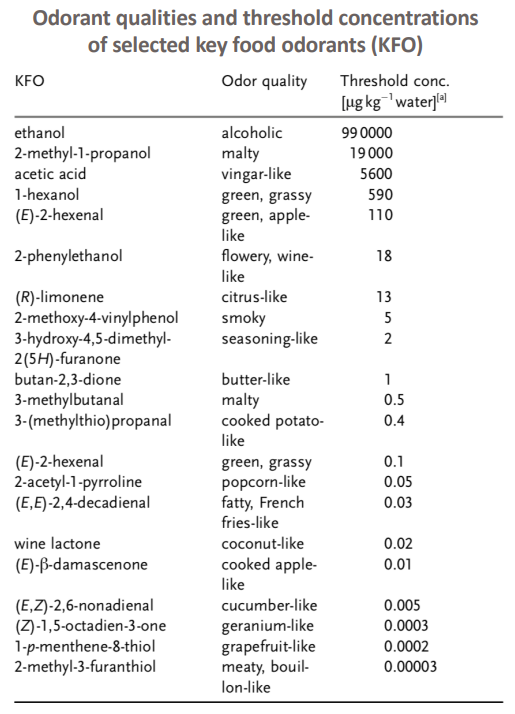 S, N 함유 향기물질은 역치가 매우 낮다 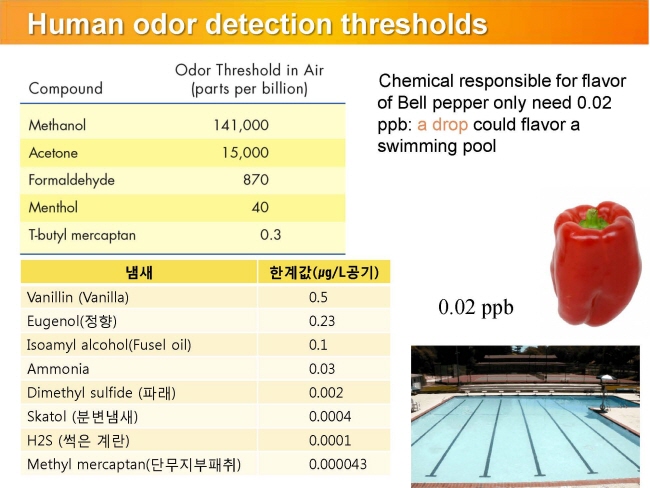 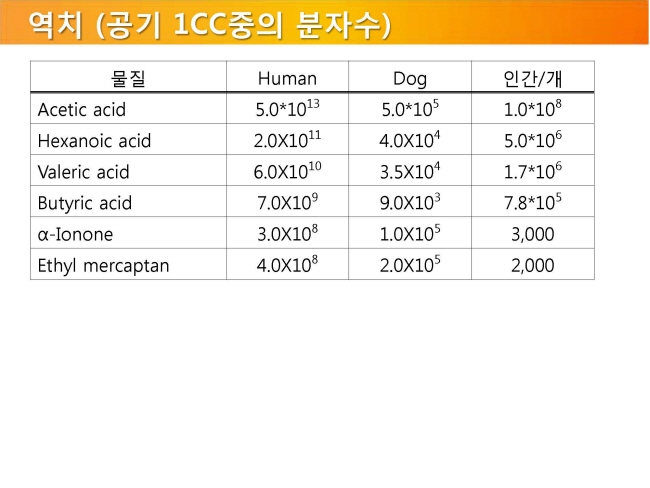 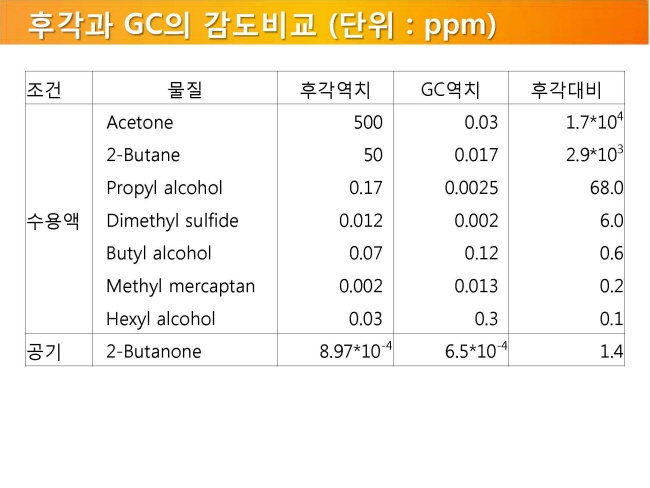 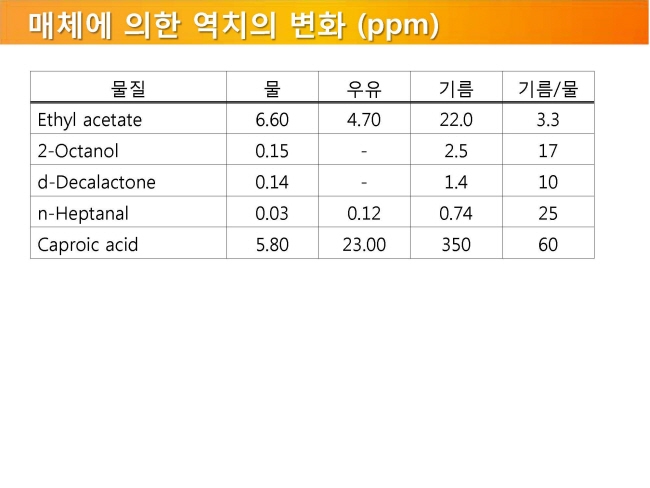 공헌도가 달라진다  『Food Chemistry』 H.D. Belitz, W. Grosch, P.Schieberle, Springer 2009 Odour thresholds for particular compounds published in the literature can vary in a broad range. As examples, odour thresholds determined in water for 3-methylbutanal (malty odour) range from 0.2 to 170 μg/L, for ethanol from 25000 to 900000 μg/L, for ethyl butanoate (fruity odour) from 0.0032 to 450 μg/L or for β-ionone (violet-like) from 0.007 to 23 μg/L (Czerny et al. 2008). For active amyl alcohol (2-methyl-1-butanol), the odour detection thresholds compiled by van Gemert (2011) from various publications are 4.15, 1.9, 0.25-0.42, 0.3, 50-60, 32.5, 1.2, 3.7 and 0.0159 mg/kg. With such variability, it is often hard to estimate an average OT value based on literature data. Such differences can be attributed to different levels of experience of the sensory panel members, to the size of the panel, as well as to contamination of the reference compounds with other odour active compounds, sometimes closely related isomers (Czerny et al. 2008). Odour thresholds and odour qualities depend on structural parameters such as chain length, unsaturation degree, double bonds position, functional groups position and molecular characteristics and stereochemistry of molecules. Physicochemical properties of volatile compounds, such as solubility, volatility, viscosity, odour etc., vary as a function of chain length. Short-chain saturated alcohols are polar solvents highly soluble in water, but when the impact of the hydroxyl group on a given alcohol structure decreases due to increasing the hydrophobic chain length (higher than C5), the ability to dissolve in water decreases; such alcohols become nonpolar. Chain length mostly affects odour threshold and aroma properties. In the case of aldehydes, compounds with low molecular weight (<150 Da) have unpleasant odours, which disappear for compounds with higher molecular weight which have sweet, fruity aromas. Also, viscosity of alcohols increases with the increasing chain length. For the 4-alkyl substituted γ-lactones an increase in alkyl chain length causes decrease in coconut aroma and an increase in fruity sweet notes. For these compounds (R)-enantiomers have more intense odours than the corresponding (S)-enantiomers (Cooke et al. 2009). Odour descriptors of R-enantiomers of γ-lactones are shown in Figure 6.1. Odour threshold (OT) is a parameter which depends not only on the length of the chain, but also on the structure of the bonds and functional groups. In saturated aldehydes OT decreases from C5 to C8, then increases. However no clear relationship has been found between chain length or bond structure and OT (Belitz et al. 2009). For straight chain acetates found as odorants in some fruits (Takeoka et al. 1996), ethyl acetate and propyl acetate have relatively high OTs (13500 and 2000 μL/103L respectively). Butyl and pentyl acetate have low OTs (respectively 66 μL/103L and 43μL/103L) which rise with increased chain length, to reach higher values (190 μL/103L for octyl acetate). For branched esters, higher OTs were noted for unsaturated compared to saturated esters. The double bond position and the configuration also influenced odour threshold. The addition of double bond in the C-2 position of acid resulted in higher OTs, whereas the addition of double bond further away from carbonyl carbon had a smaller effect on OT (Takeoka et al. 1998). Addition of double bond to a structure of 2-acetyl-1-pyrroline and changing positions of double bonds, which results in a structurally similar 2-acetyl-pyrrole, caused a drastic difference in OTs of these compounds as for 2-acetyl-1-pyroline it was estimated at 0.1 μg/L, whereas for 2-acetylpyrrole it was 170 000 μg/L (Buttery 1999). A change of the double bond configuration or functional group’s position influences the odour threshold or aroma quality of a given compound, but these properties depend also on the size of the molecule. For example, changing the bond of a molecule from saturated to unsaturated may result in modifying aroma quality in small molecules, as illustrated by trans-2-hexenal which has a herbal green-like aroma while saturated hexanal has a fruity, strawberry-like odour. On the other hand, in larger molecules, these changes affect only the odour threshold of the compound. The effect of removing or changing the hydroxyl group in aliphatic or aromatic alcohols often leads to the disappearance of odour (Reineccius 2005). Comparing odour thresholds between cis and trans isomers of alka-1,5-dien-3-ones and alka-1,5-dien-ols shows that few differences are observed for long chain (C14) homologues whereas, for octadienones and octadienols significant differences exist. (Z)-octa-1,5-dien-3-one (geranium-like metallic odour) has an odour threshold of 0.003 ng/L (air), whereas its corresponding (E)-octa-1,5-dien-3-one (geranium-like odour) has an odour threshold of 1.4 ng/L (air). Similarly, (Z)-octa-1,5-dien-3-ol (geranium-like) has an odour threshold of 1.3 ng/L, whereas its (E) isomer (geranium-like, green odour) has an odour threshold of 36.2 ng/L (Lorber et al. 2014). Odour thresholds of compounds of similar structures with different functional groups locations can differ substantially. As shown in Figure 6.2, 2-Ethyl-3,5-dimethyl pyrazine and 2-ethyl-3,6-dimethyl pyrazine differ in position of one of the methyl groups only, which makes them similar in retention on nonpolar GC columns, however their odour thresholds in water differ over 200 times: 8.6 μg/L for 2-ethyl-3,6-dimethylpyrazine, and 0.04 μg/L for 2-ethyl-3,5-dimethylpyrazine (Buttery and Ling 1997a). An additional alkylation on 4-methylphenol in ortho- and para- position respectively modifies the odour threshold of the corresponding molecules to 27 ng/L for 2,4-dimethylphenol and 143 ng/L for 3,4-dimethylphenol which corresponds to an increase by factors of 225 and 1190 respectively. Trimethyl phenols are also characterised by lower odour thresholds than dimethylphenols (Czerny et al. 2011). Other examples of large differences in odour thresholds of structurally similar earthy/musty smelling pyrazines are given in Figure 6.2. 2-Ethenyl-3-ethyl-5-methylpyrazine has an odour threshold of 0.014 ng/L in air, whereas 3-ethenyl-2-ethyl-5-methylpyrazine’s odour threshold in air is 109.5 ng/L (Czerny et al. 1996). Also 2-methoxy-3,5-dimethylpyrazine (earthy smelling) and 3-methoxy-2,5-dimethylpyrazine (pea-like smelling) have very different odour thresholds of 0.001 ng/L (air) and 56 ng/L respectively (Molyneux and Schieberle 2007). From the above mentioned examples of pyrazines it is evident that trace amounts of another more potent isomer can drastically influence the odour threshold of analysed compound and that the great chemical similarity of isomers with different odour threshold is an additional challenge for the separation of these compounds by GC and in consequence also by GC-O. Chirality influences both odour quality and odour threshold of aroma compounds (Brenna et al. 2003). In the field of food flavours, there are well known examples of different odour notes of enantiomers: carvone (caraway (+) and spearmint (−)), nootkatone (grapefruit (+) and woody/spicy (−)), limonene (orange (+) and turpentine (−)), menthol (vegetable/mild mint (+) and strong mint/cooling (−)), or linalool (sweet/petitgrain (+) and woody/lavender (−)). The enantiomers of whisky (or quercus) lactones (Figure 6.3), which are extracted from oak barrels in which alcoholic beverages are kept, have been described with different odour notes (Brown et al. 2006). Their natural enantiomeric excesses are 77% for (3S, 4S)-lactone and 23% for (3S, 4R)-lactone. Odour descriptors for (3S, 4S) enantiomer are weak coconut, earthy, mouldy, hay, whereas for (3S, 4R) they are spicy, celery, weak coconut, distinct green walnut. The remaining enantiomers have sweet, fresh, bright, coconut notes (3R, 4R), and an intense coconut note with an after odour reminiscent of celery (3R, 4S). The odour thresholds in white wine of naturally occurring isomers, (3S,4S) cis-oak lactone and (3S, 4R) trans-oak lactone, are 20 and 140 μg/L respectively, whereas those of the two non-naturally occurring isomers, (3R, 4R)-cis- and (3R 4S)-trans, are 132 and 330 μg/L respectively (Brown et al. 2006). Guth (1996) identified in white wines a sweet, coconut smelling wine lactone (3a,4,5,7a-tetrahydro-3,6-dimethylbenzofuran-2(3H)-one). The configuration of this naturally occurring wine lactone was established as (3S,3aS,7aR) enantiomer. All possible enantiomers were synthesized and odour thresholds estimated for these compounds (Figure 6.4). The lowest odour threshold (0.02 pg/L in air, range of 0.01-0.04 pg/L) was noted for the naturally occurring isomer while the others exhibited odour thresholds ranging from 0.007 to >1000 ng/L in air (Guth 1996). Aroma perception is not only due to the amount of odorant but also influenced by the matrix from which aroma compounds are released into the vapour phase. Interactions between the components matrix and odorants (discussed in detail in Chapter 9) influence odorants solubility and as a result their vapour/matrix partition coefficients. For example for apple odorants, the ratio of odour thresholds in juice vs. in water can vary from 5.2 (determined for butyl butanoate) to 600 for ethyl 2-methyl butanoate (Cliff et al. 2011). Oak lactones odour thresholds in wine differ between white and red wines: in white wine (3S, 4S) cis and (3S, 4R) trans isomers have odour thresholds of 20 and 140 μg/L respectively, whereas in red wine they are 54 and 370 μg/L respectively (Brown et al. 2006). As summarised by Mazzoleni and Maggi (2007), odour thresholds for trichloroanisole (2,4,6-TCA) differ substantially from 0.03 ng/L in water, 0.8 ng/L in mineral water, 4 ng/L in dry white wine to 22 ng/L in red wine 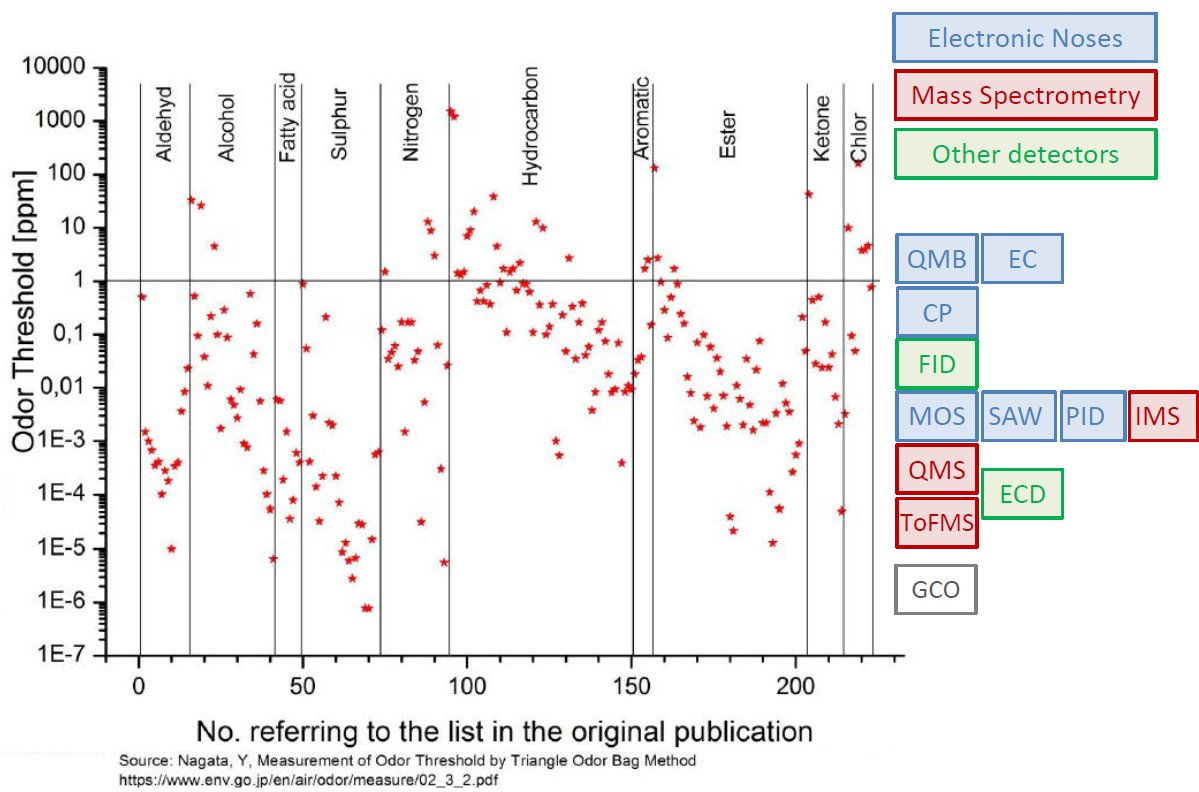 |
||||
|
|
|||