| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
내몸 ≫ 신경전달 ≫ 신경세포 신경교세포 : Astrocyte 세포종류 - 신경세포 + 글리아 - 글리아 = Oligodendrocyte + Astrocyte 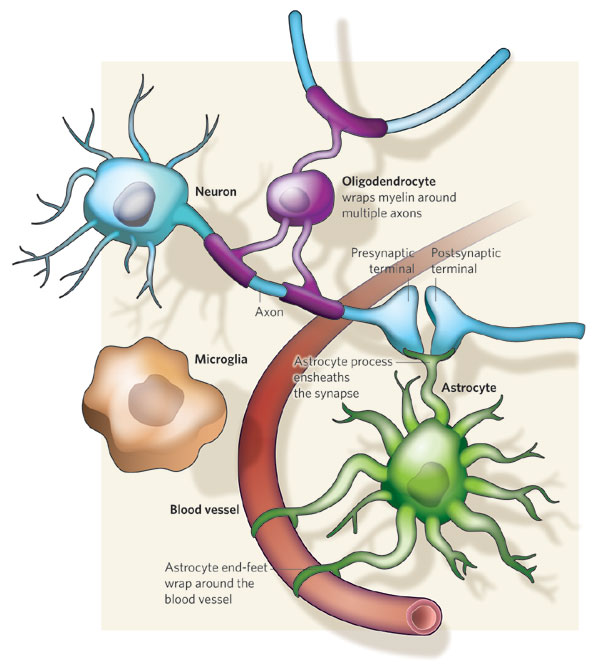 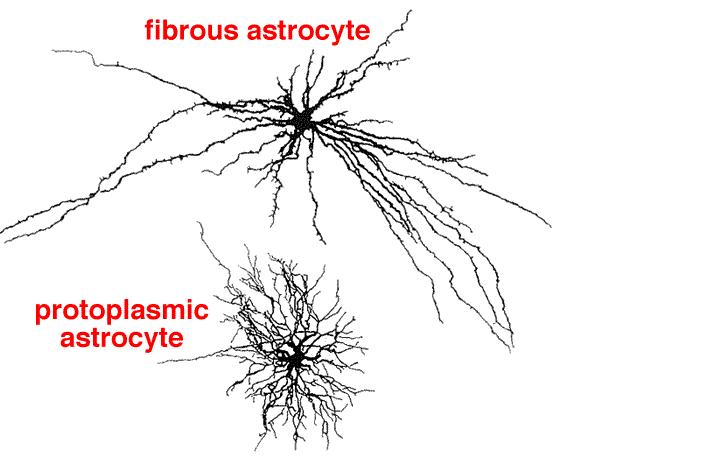 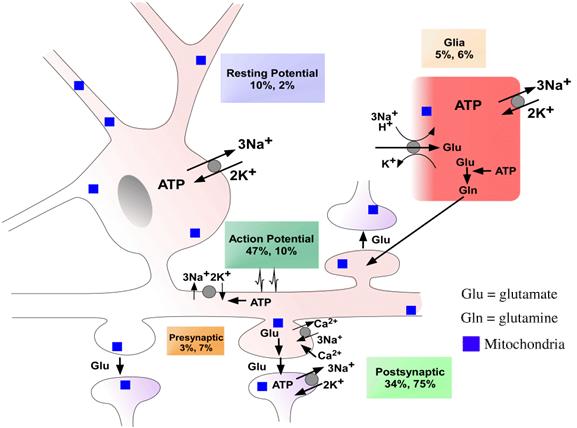 Astrocytic/neuronal glucose oxidation and lactate shuttle. The biological interactive compartments include the blood vessel (right panel) , the synapse with the afferent and efferent neurons (left panels), and the astrocyte (central panel) with a typical end feet in connexion with the blood vessel and an extension which envelops the synapse and contributes to the tripartite synapse, a current trend in neurobiology. Blood glucose may enter the astrocyte via endothelial and astrocytic glucose transporters 1 (GLUT1), the entry in the neuron further requiring neuronal glucose transporter 3 (GLUT3). Within the astrocyte, part of glucose may be stored as glycogen via glycogenesis (event not shown on the figure) whereas conversion to pyruvate occurs via cytosolic glycolysis. This glucose oxidation results in NADH and ATP productions. To procede to completion, astrocyte glycolysis needs re-cycling of cytosolic NAD+ and ADP. Recovery of astrocyte cytosolic NAD+ is ensured by LDH5 (Lactate DeHydrogenase 5) which converts pyruvate to lactate further exported in the neuron via astrocytic MCT1 (MonoCarboxylate Transporter 1) and neuronal MCT2 in which metabolism is directed towards mitochondrial pyruvate oxidation. Recovery of ADP can occur during glutamatergic neurotransmission according to events depicted in figure 2.Astrocyte to neuron shuttle of lactate occurs for both pre-synaptic (illustrated) and post-synaptic (not shown) neurons. This type of shuttle classically involves an extracellular pool of metabolites (astrocytes → extracellular space → neuronal shuttle). Other comments are in the text 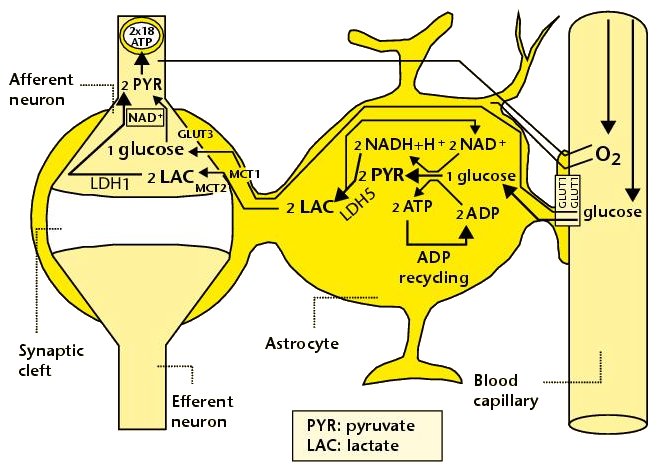 Glutamate-induced astrocyte cytosolic ADP formation and subsequent glycolysis activation - Mechanisms leading to an increased ADP/ATP ratio in the astrocyte cytosol, a metabolic event which stimulates local glycolysis, involve the release of glutamate in the synaptic cleft with consequent activation of post-synaptic and astrocytic membrane ionotropic (NMDA and AMPA receptors) and metabotropic receptors and subsequent removal of glutamate mainly by astrocytic glutamate uptake catalyzed by EAAT 1, 2 and/or 4 (Excito Amino Acid Transporters 1, 2 and/or 4) and to a little extent by pre-synaptic EAAT 3, 4 and/or 5. One glutamate is up-taken by astrocyte EAATs in the same time as 3 Na+. Further astrocytic disposition of both the metabolite and the electrolyte generates ADP from ATP through reactions catalyzed by glutamine synthase (GS) and Na/K.ATPase (ATPase), respectively. This gives rise to a huge increase in astrocyte cytosolic ADP/ATP ratio which results in a boosting of astrocyte glycolysis.Other comments are in the text. 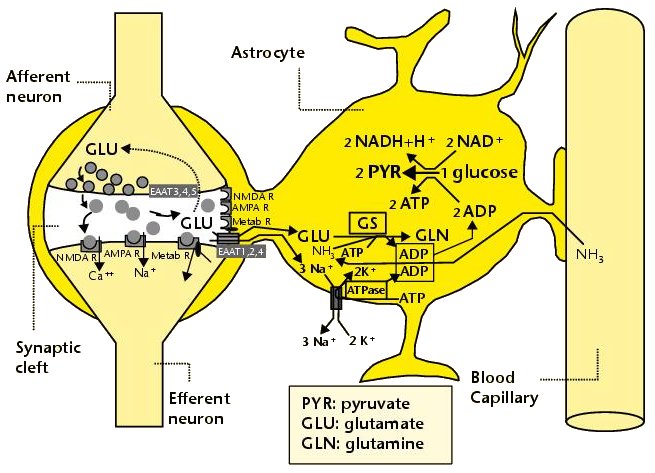 Glutamate-mediated neurovascular coupling. As illustrated on Figure 2, glutamate via induction of a high astrocytic cytosolic ADP/ATP ratio can boost astrocyte glycolysis. This leads to a stimulation of events depicted in figure 1, namely increased astrocyte pyruvate and lactate formation rates and hence increased lactate supply to neurons with enhanced neuronal metabolism. The metabolic/energetic astrocyte activation needs concomitantly some adaptation in the local blood/astrocyte exchanges in order to provide substrates and to remove metabolic end-products in an adequate way. This figure 3 illustrates mechanisms allowing glutamate released in the synaptic cleft to induce this local vasodilatation through convergent routes involving activation of post-synaptic and astrocytic glutamate receptors and subsequent rise in cytoplasmic Ca++ concentration.Glutamate activation of post-synaptic receptors leads to increased cellular uptake (NMDA receptor) and increased intracellular mobilization (stimulation of metabotropic receptors causing release of inositol-triphosphate [IP3] from the phospho-inositol bisphosphate [PIP2] moiety of membrane phospholipids mobilizes cellular calcium stores) of Ca++. Comparable mechanisms also mediate a glutamate-induced rise in astrocytic cytoplasmic Ca++ levels. In the postsynaptic neuron, the enhanced Ca++ levels activate neuronal oxide synthase (nNOS) and hence increase ●NO formation from arginine. The nitric oxide synthase (NOS) of the astrocyte is also activated by cytoplamsic rise in Ca++. The enhanced ●NO formation originating from both the postsynaptic neuronal and astrocytic metabolism is a first mechanism for blood vessel relaxation (note that ●NO is a highly diffusible gas compound and its relaxing properties on the vasculature results from its interaction with guanylate cyclase). A second mechanism holds in the activation of astrocytic phospholipase A2 (PLA2) which stimulates release from membrane phospholipids of arachidonic acid. Upon action of cytochrome P450 (cyt P450) and cyclooxygenase 2 (COX2), arachidonate gives rise to EETs (epoxyeicosatrienoic acids) and prostaglandin E2 (PGE2), vasoactive metabolites inducing additional blood vessel relaxation. 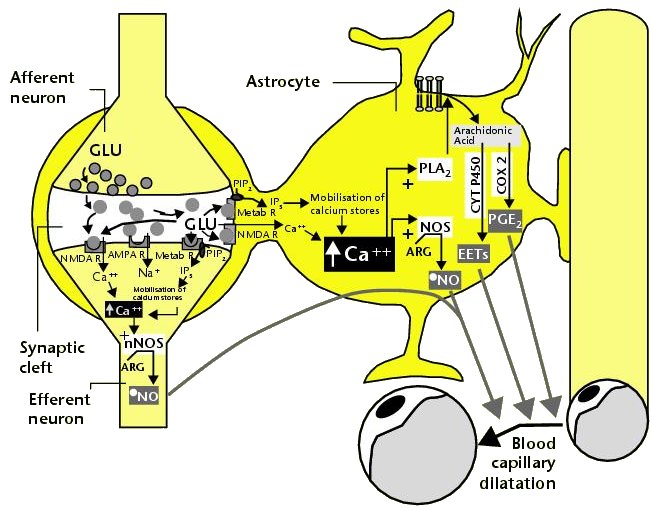 Ketogenic diet – induced mechanisms that increase GABA synthesis and neurotransmission in brain. Ketone bodies supplied to neurons are accounted for by two metabolic routes, blood ketones of hepatic origin and astrocytic ketone body formation from blood fatty acids, without considering what route is here prominant. Neuronal mitochondrial oxidation of ketones to acetyl-CoA has been proposed to mobilize oxaloacetate in the Kreb’s cycle at the step catalysed by citrate synthase (CS). This induces a shift in the equilibrium of the reaction catalysed by aspartate-glutamate transaminase (AGT) in favour of glutamate formation. Subsequent stimulation of glutamate decarboxylation to GABA is catalysed by GAD (Glutamic Acid Decarboxylase) and leads to enhanced GABA synthesis and neurotransmission as a brain anticonvulsant mechanism. 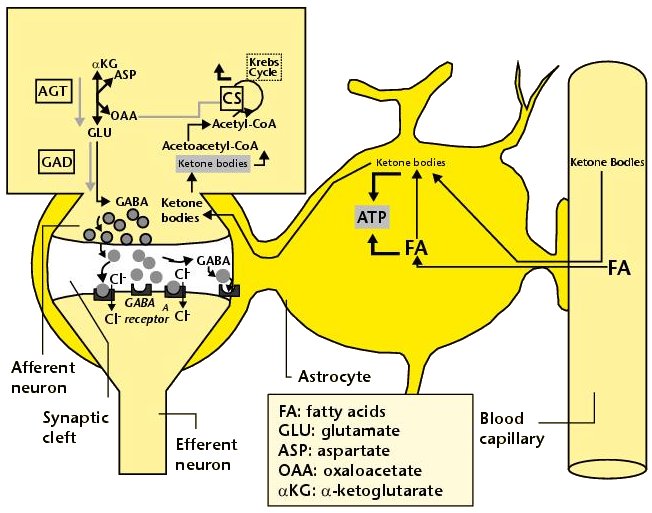 Ketogenic diet (and starvation) – induced mechanisms that lower glutamate neurotransmission in brain. Although upon ketogenic diet (and starvation) a larger portion of glutamate is converted to GABA in the brain (figure 4), a part of glutamate and/or glutamine escapes the glutamate/glutamine cycle and may be lost or removed from the astrocyte to blood. In these diet conditions, more astrocytic glutamine would be removed in blood by exchange with leucine whose blood levels are increased in starvation [24] and more glutamate would also be lost as a result of increased brain to blood ratio affecting selectively alanine having a push effect on removal of this aminoacid in blood and hence on pyruvate transmination to alanine, a conversion proposed to be associated to glutamate consumption 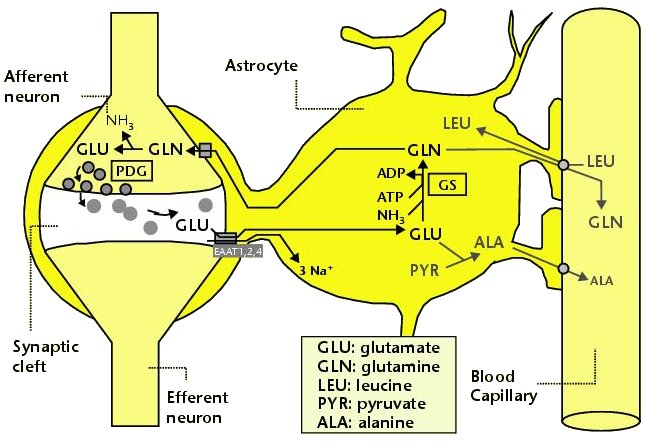
|
||||
|
|
|||