| Hint | Food | 맛과향 | Diet | Health | 불량지식 | 자연과학 | My Book | 유튜브 | Frims | 원 료 | 제 품 | Update | Site |
|
질병 ≫ 암 ≫ 발암물질 발암성 : 효소에 의한 전환, 에폭시 에폭사이드 : 활성산소 발생 - 아플라톡신, 벤조피렌, 아크릴아미드 - 철분(Fe) 함유 효소 : 시토크롬 P450 활성산소 : 노화와 질병의 주범 - 체내 산화와 환원 - 항산화제 역할 : 항산화제의 부작용 - 미토콘드리아와 활성산소 - 제초제 그라목손 : 최악의 활성산소 유발물질 아플라톡신이 발암물질이라는 것은 정말 많이 들었다. 그런데 그것이 왜 발암물질로 작용하는지는 모른다. 그 자체는 발암물질이 아닌데 내 몸의 효소에 의해 발암물질로 바뀐다 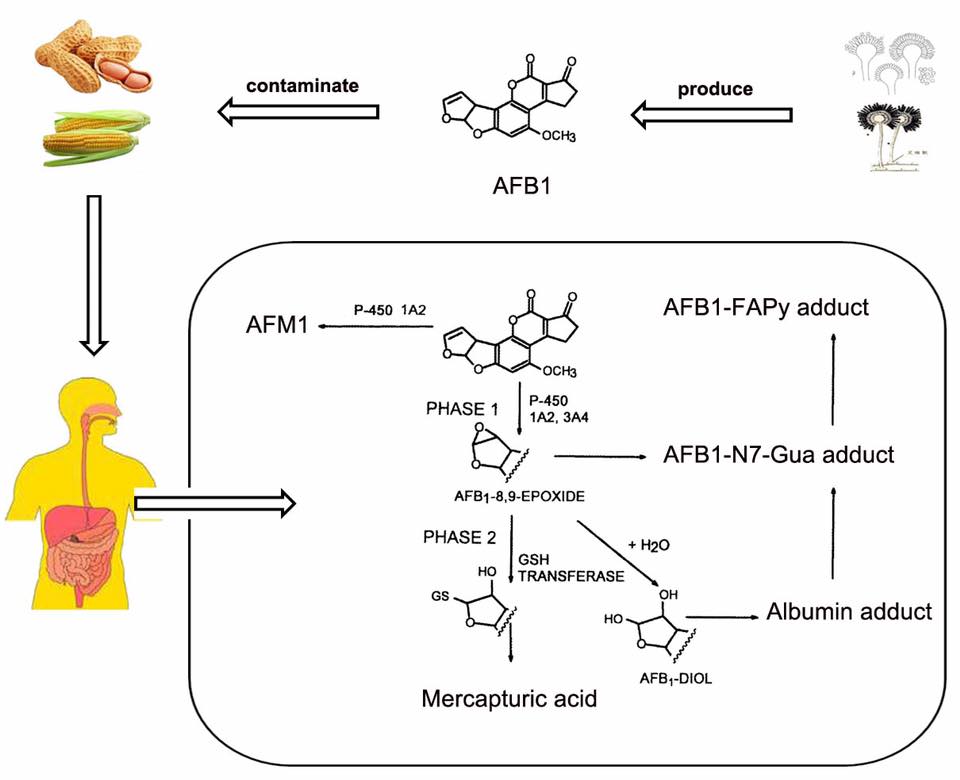 International sources of commercial peanut butter, cooking oils (e.g. olive, peanut and sesame oil), and cosmetics have been identified as contaminated with aflatoxin.[29][30][31] In some instances, liquid chromatography-tandem mass spectrometry (LC-MS/MS), and other analytical methods, revealed a range from 48% to 80% of selected product samples as containing detectable quantities of aflatoxin. In many of these contaminated food products, the aflatoxin exceeded the safe limits of the U.S. Food and Drug Administration (FDA), or other regulatory agency In dogs, aflatoxin has potential to lead to liver disease. Low levels of aflatoxin exposure require continuous consumption for several weeks to months in order for signs of liver dysfunction to appear.[21] Some articles have suggested the toxic level in dog food is 100–300 ppb and requires continuous exposure or consumption for a few weeks to months to develop aflatoxicosis.[22] No information is available to suggest that recovered dogs will later succumb to an aflatoxin-induced disease. Turkeys are extremely susceptible to aflatoxicosis. Recent studies have revealed that this is due to the efficient cytochrome P450 mediated metabolism of aflatoxin B1 in the liver of turkeys and deficient glutathione-S-transferase mediated detoxification.[23][24] Some studies on pregnant hamsters showed a significant relationship between exposure of aflatoxin B1 (4 mg/kg, single dose) and the appearance of developmental anomalies in their offspring 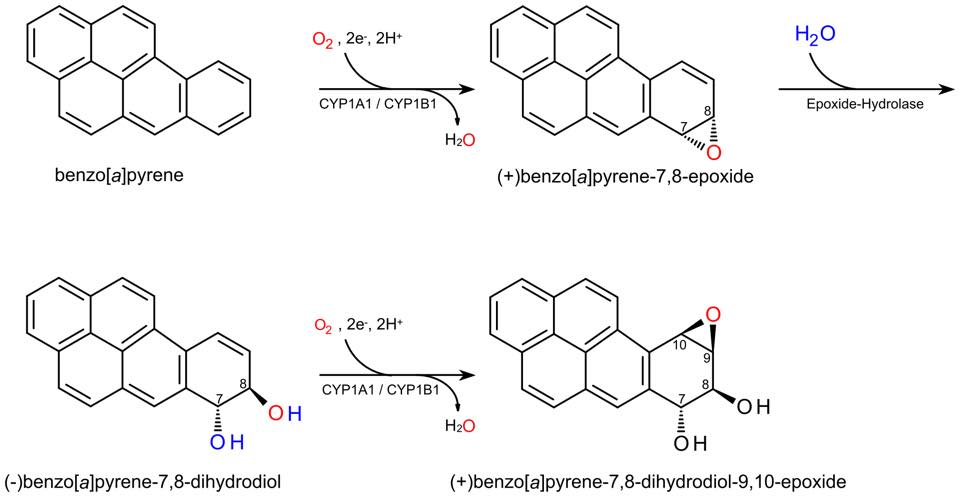 Properly speaking, Benzo(a)pyrene(BaP) is a procarcinogen, meaning that its mechanism of carcinogenesis depends on its enzymatic metabolism to BaP diol epoxide. It intercalates in DNA, covalently bonding to the nucleophilic guanine bases. X-ray crystallographic and nuclear magnetic resonance structure studies have shown how this binding distorts the DNA[21] by confusing the double-helical DNA structure. This disrupts the normal process of copying DNA and causes mutations, which explains the occurrence of cancer after exposure. This mechanism of action is similar to that of aflatoxin which binds to the N7 position of guanine.[22] There are indications that benzo[a]pyrene diol epoxide specifically targets the protective p53 gene.[23] This gene is a transcription factor that regulates the cell cycle and hence functions as a tumor suppressor. By inducing G (guanine) to T (thymidine) transversions in transversion hotspots within p53, there is a probability that benzo[a]pyrene diol epoxide inactivates the tumor suppression ability in certain cells, leading to cancer. Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is the carcinogenic product of three enzymatic reactions:[24] Benzo[a]pyrene is first oxidized by cytochrome P450 1A1 to form a variety of products, including (+)benzo[a]pyrene-7,8-epoxide.[25] This product is metabolized by epoxide hydrolase, opening up the epoxide ring to yield (-)benzo[a]pyrene-7,8-dihydrodiol. The ultimate carcinogen is formed after another reaction with cytochrome P4501A1 to yield the (+)benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. It is this diol epoxide that covalently binds to DNA. BaP induces cytochrome P4501A1 (CYP1A1) by binding to the AHR (aryl hydrocarbon receptor) in the cytosol.[26] Upon binding the transformed receptor translocates to the nucleus where it dimerises with ARNT (aryl hydrocarbon receptor nuclear translocator) and then binds xenobiotic response elements (XREs) in DNA located upstream of certain genes. This process increases transcription of certain genes, notably CYP1A1, followed by increased CYP1A1 protein production.[26] This process is similar to induction of CYP1A1 by certain polychlorinated biphenyls and dioxins. Seemingly, CYP1A1 activity in the intestinal mucosa prevents major amounts of ingested benzo(a)pyrene to enter portal blood and systemic circulation.[27] Intestinal, but not hepatic, expression of CYP1A1 depends on TOLL-like receptor 2 (TLR2),[28] which is a eucaryotic receptor for bacterial surface structures such as lipoteichoic acid. 아크릴아미드의 에폭사이드 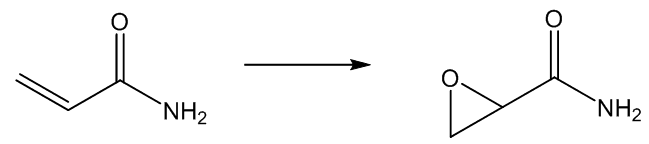 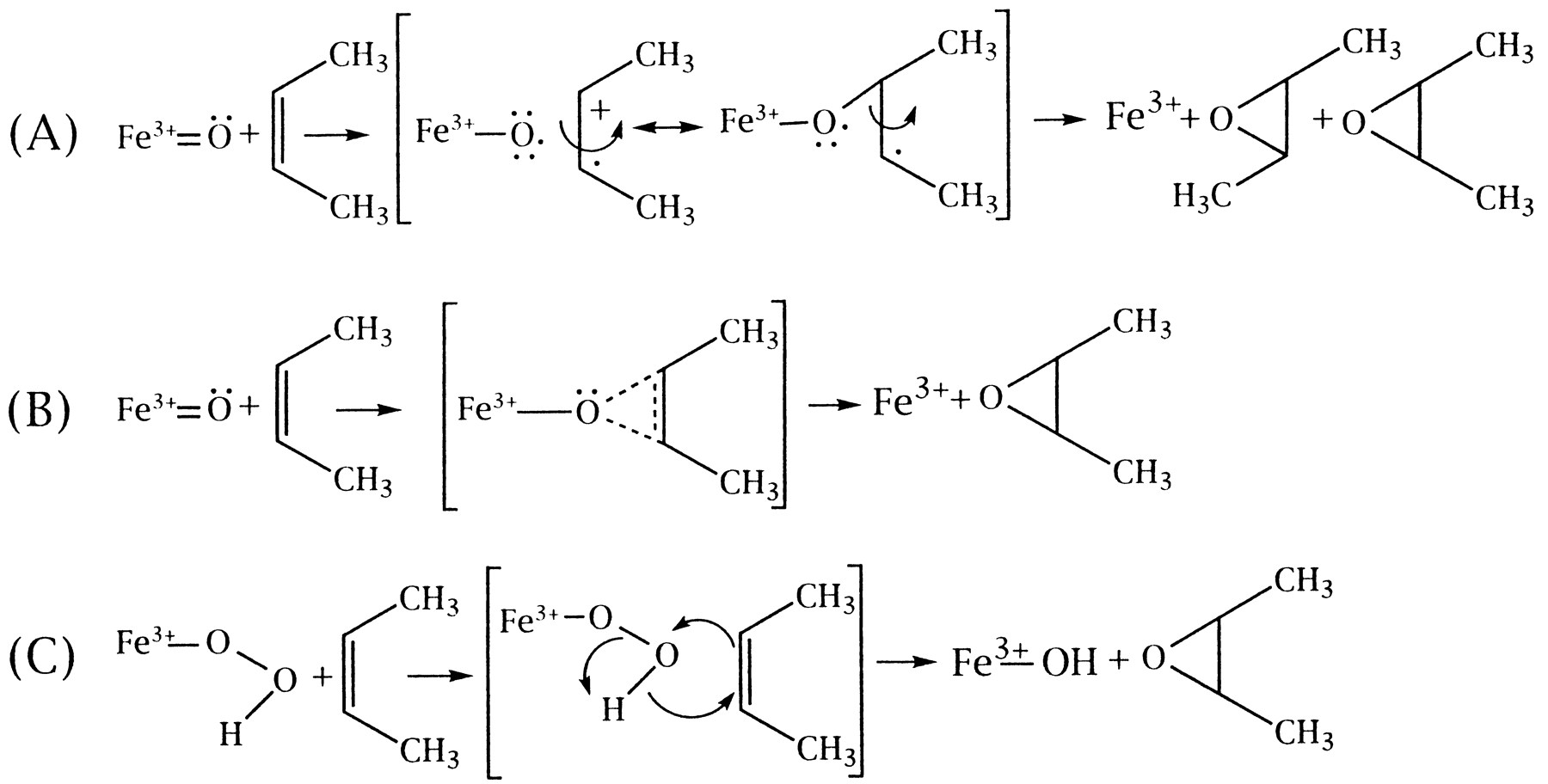  |
||||
|
|
|||